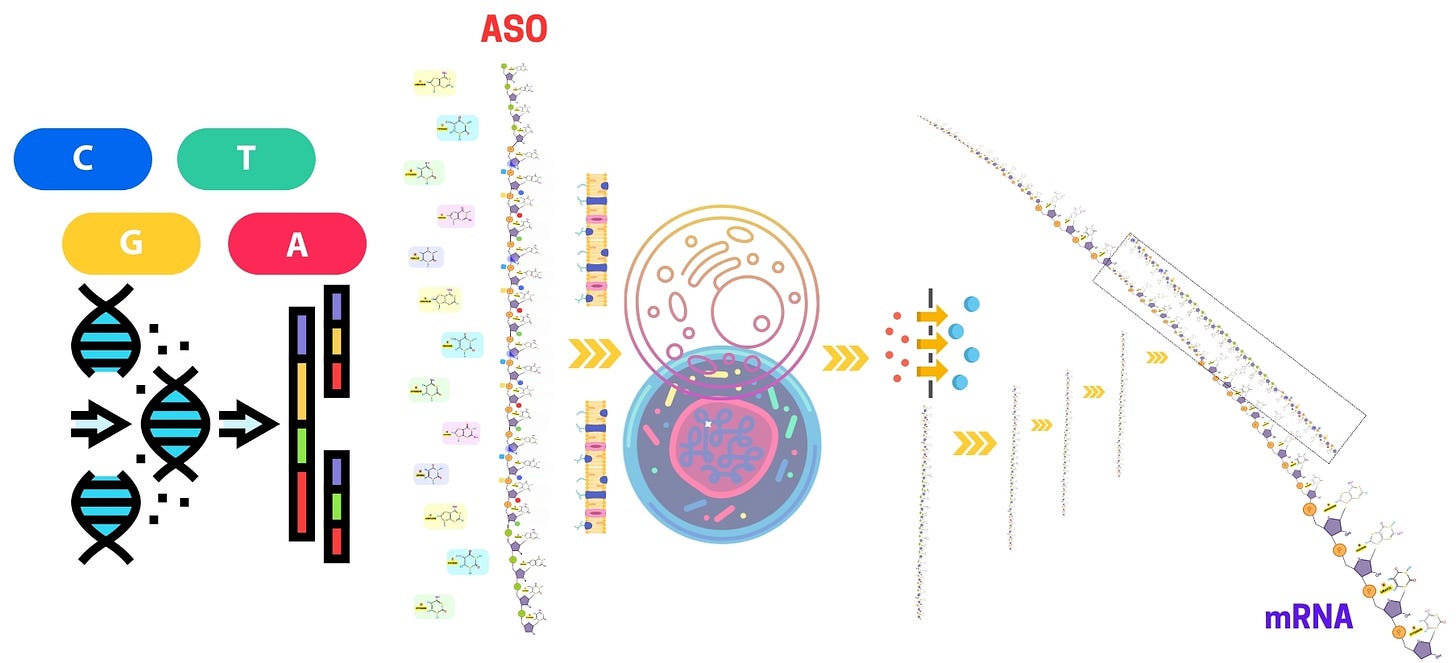
Antisense Oligonucleotides - Nanocarrier Integration in ASO Delivery
Luke McLaughlin, Scientific Digital Marketing, Synthetic Biology, Nucleic Acid Therapeutics and Antibody Engineering, Biotech Writer
The integration of nanocarriers in antisense oligonucleotide (ASO) delivery has revolutionized the stability, targeting, and efficiency of antisense oligonucleotide therapy for genetic disorders, neurodegenerative diseases, and cancer. Nanocarrier-based systems enhance the bioavailability and cellular uptake of ASOs, overcoming challenges such as enzymatic degradation, poor membrane permeability, and off-target effects.
During antisense oligonucleotide synthesis and purification, chemical modifications like phosphorothioate oligonucleotides, PMO oligos, and gapmer antisense oligonucleotides improve nuclease resistance and increase therapeutic potency. The use of locked nucleic acid (LNA) antisense oligonucleotides and morpholino antisense oligos further enhances target affinity, ensuring efficient RNA hybridization. Additionally, 2'-O-methyl phosphorothioate ASOs are commonly integrated into nanocarrier systems to improve RNA targeting efficiency and reduce off-target interactions.
Nanocarrier systems, such as lipid nanoparticles (LNPs), polymeric nanoparticles, liposomes, and dendrimers, serve as advanced delivery vehicles for ASOs. These systems protect ASOs from degradation while enabling controlled release and targeted gene silencing in in vitro and in vivo models. In antisense RNAi screening, nanocarrier-based ASO formulations are evaluated for their cellular uptake, endosomal escape, and efficiency in gene knockdown. Additionally, surface modifications with targeting ligands enhance ASO biodistribution, enabling precise delivery to diseased tissues.
The incorporation of nanocarriers in antisense oligonucleotide delivery represents a major advancement in ASO-based therapeutics, facilitating systemic administration while minimizing adverse effects. Ongoing innovations in nanotechnology and antisense oligonucleotide synthesis and purification continue to refine these delivery strategies, ensuring that ASOs reach their full potential in treating diseases with unmet medical needs.
The core focus of this article will be:
Role of nanocarriers in enhancing ASO delivery and stability.
Types of nanocarriers: lipid-based, polymer-based, inorganic, and hybrid.
Targeted delivery using active (ligand-based) and passive (EPR effect) mechanisms.
The therapeutic potential of antisense oligonucleotides (ASOs) in gene modulation has been well-established across a wide range of genetic diseases. However, despite their high specificity in targeting mRNA, ASOs face significant challenges related to delivery, stability, and cellular uptake. Their large molecular size, negative charge, and susceptibility to rapid nuclease degradation severely limit their bioavailability, making efficient delivery into target cells a major obstacle in clinical applications. Furthermore, achieving effective ASO concentrations within specific tissues while minimizing off-target effects and systemic toxicity remains a challenge in the field of oligonucleotide therapeutics. To address these issues, the integration of nanocarrier systems has emerged as a transformative approach for optimizing ASO delivery.
Nanocarriers serve as vehicles that protect ASOs from degradation, enhance their transport across biological barriers, and improve their accumulation in target tissues. These delivery platforms can be engineered to encapsulate or complex with ASOs, offering enhanced stability, efficient uptake, and controlled release of the ASOs within target cells. Nanocarriers improve the pharmacokinetic properties of ASOs, extending their circulation time and reducing renal clearance, while simultaneously mitigating off-target effects by directing ASOs to specific tissues or cells. By leveraging both passive and active targeting mechanisms, nanocarriers can increase the therapeutic precision of ASO therapies.
Lipid-based nanocarriers, such as lipid nanoparticles (LNPs), are among the most commonly used systems for ASO delivery due to their ability to encapsulate nucleic acids, protect them from nucleases, and facilitate endosomal escape after cellular uptake. Polymer-based systems, including polymeric micelles and dendrimers, offer additional versatility, allowing for tunable release profiles and functionalization with targeting ligands that enhance delivery to specific cells or tissues. Inorganic nanocarriers, such as gold nanoparticles (AuNPs) and mesoporous silica nanoparticles (MSNs), provide stable platforms for ASO conjugation, offering additional features such as real-time tracking of ASO delivery and controlled release based on environmental stimuli. Hybrid nanocarriers, which combine the benefits of lipid, polymeric, and inorganic materials, further optimize ASO delivery by enhancing stability, reducing toxicity, and improving biodistribution.
One of the key advantages of nanocarriers is their ability to achieve targeted delivery through both passive and active mechanisms. Passive targeting, exemplified by the Enhanced Permeability and Retention (EPR) effect, exploits the leaky vasculature and poor lymphatic drainage characteristic of tumors and inflamed tissues. This allows nanocarriers to preferentially accumulate in these regions, leading to higher concentrations of ASOs in diseased tissues without the need for specific targeting ligands. On the other hand, active targeting relies on the functionalization of nanocarriers with ligands, such as peptides, antibodies, or small molecules, that recognize and bind to receptors or antigens specifically expressed on target cells. This approach enables precise ASO delivery to particular tissues, such as tumor cells or hepatocytes, while minimizing off-target effects in healthy tissues.
Nanocarriers also play a crucial role in overcoming one of the primary challenges in intracellular delivery: endosomal escape. Once nanocarriers are taken up by cells through endocytosis, ASOs often become trapped in endosomal compartments, limiting their ability to reach the cytoplasm or nucleus where they exert their therapeutic effects. Nanocarrier systems, such as lipid nanoparticles and polyethyleneimine (PEI)-based nanoparticles, are designed to facilitate endosomal escape through mechanisms such as the proton sponge effect or pH-sensitive lipid destabilization. These mechanisms promote the release of ASOs from the endosomes into the cytoplasm, significantly enhancing their bioavailability and therapeutic efficacy.
Additionally, nanocarriers improve the pharmacokinetics of ASOs by extending their half-life in circulation, reducing their rapid renal clearance, and increasing their accumulation in target tissues. Surface modifications, such as PEGylation (the attachment of polyethylene glycol chains), are frequently employed to create a hydrophilic barrier that reduces immune recognition and opsonization, thus prolonging the circulation time of the ASO-nanocarrier complex. This reduces the need for frequent dosing and enhances the overall therapeutic efficacy of ASO-based treatments.
In vivo studies have demonstrated the effectiveness of nanocarriers in improving the biodistribution and therapeutic outcomes of ASO treatments. Nanocarriers can be tracked and imaged in vivo using fluorescent or radioactive labels, allowing researchers to monitor their distribution, clearance, and accumulation in real-time. This ability to visualize the pharmacokinetics of nanocarrier-based ASO delivery provides critical data for optimizing dosing regimens and improving therapeutic outcomes.
In this article, we will delve into the technical aspects of nanocarrier integration in ASO delivery, exploring the various types of nanocarriers, including lipid-based, polymer-based, inorganic, and hybrid systems, and their specific roles in enhancing ASO stability, delivery, and bioavailability. We will also examine the mechanisms of targeted delivery through active ligand-based targeting and passive strategies like the EPR effect. Finally, we will discuss how nanocarriers facilitate endosomal escape and improve the pharmacokinetics and in vivo efficacy of ASOs. The integration of nanocarrier technology represents a significant advancement in the field of ASO therapeutics, enabling the development of more efficient, targeted, and clinically viable gene therapies for a wide range of diseases.
Nanocarrier Integration in ASO Delivery
Nanocarriers play a pivotal role in optimizing the delivery of ASOs, particularly in overcoming challenges related to cellular uptake and stability. During in vitro testing, nanocarriers, such as lipid nanoparticles or polymeric nanoparticles, can significantly enhance the efficiency of ASO delivery by facilitating cellular uptake and protecting the ASO from degradation. These nanocarriers are engineered to efficiently transport ASOs into cells and even specific subcellular compartments, such as the nucleus or cytoplasm, where the ASO can exert its therapeutic effects.
Nanocarriers have emerged as a pivotal tool for improving the delivery of antisense oligonucleotides (ASOs). They play a critical role in enhancing cellular uptake, increasing ASO stability, and ensuring targeted delivery to specific tissues or cell types. By addressing the challenges of poor bioavailability, rapid degradation, and off-target effects, nanocarriers greatly improve the pharmacokinetic properties and therapeutic efficacy of ASOs. In this section, we will explore the technical aspects of nanocarrier design, types, and mechanisms of action, as well as their integration into ASO delivery systems.
Challenges in ASO Delivery and the Role of Nanocarriers
ASOs face several key challenges in reaching their intracellular targets, including:
Poor Membrane Permeability: ASOs, due to their large size and negative charge, cannot easily cross cell membranes.
Nuclease Degradation: ASOs are rapidly degraded by nucleases present in biological fluids, reducing their therapeutic efficacy.
Off-Target Effects: Without precise targeting, ASOs may accumulate in non-target tissues, leading to off-target effects and potential toxicity.
Limited Bioavailability: Systemic delivery of ASOs can result in suboptimal tissue distribution, with only a fraction of the ASO reaching the intended target tissue or cell type.
Nanocarriers help address these challenges by enhancing the delivery of ASOs to target cells and tissues, protecting them from degradation, and improving their overall pharmacokinetic properties.
Types of Nanocarriers for ASO Delivery
Various nanocarrier systems have been developed to deliver ASOs effectively. These include lipid-based, polymer-based, inorganic, and hybrid nanocarriers, each offering unique advantages for improving ASO delivery.
Lipid-Based Nanocarriers
Lipid-based nanocarriers are the most widely used systems for ASO delivery due to their biocompatibility, ability to encapsulate nucleic acids, and high transfection efficiency.
Lipid Nanoparticles (LNPs) Structure: LNPs are spherical vesicles consisting of a lipid bilayer or monolayer that encapsulates ASOs within their aqueous core. They are often formulated from ionizable lipids, which are neutral at physiological pH but become positively charged in acidic environments (e.g., within endosomes), promoting endosomal escape. Endosomal Escape: One of the main advantages of LNPs is their ability to escape the endosomal compartment after cellular uptake. Upon encountering the acidic environment of the endosome, ionizable lipids in LNPs become protonated, destabilizing the endosomal membrane and releasing the ASO into the cytoplasm. PEGylation: LNPs are often PEGylated (coated with polyethylene glycol) to increase circulation time and reduce immune recognition. PEGylation reduces protein adsorption and opsonization, prolonging the half-life of the LNPs in the bloodstream and improving tissue distribution.
Cationic Liposomes Mechanism: Cationic liposomes are composed of positively charged lipids, such as DOTAP (dioleoyl trimethylammonium propane), which form electrostatic complexes with negatively charged ASOs. The positive charge facilitates interaction with the negatively charged cell membrane, enhancing uptake through endocytosis. Limitations: While cationic liposomes improve ASO delivery, their strong positive charge can lead to toxicity and immune activation. Therefore, careful optimization of lipid composition is required to balance efficacy and safety.
Solid Lipid Nanoparticles (SLNs) Composition: SLNs consist of a solid lipid core that is stabilized by surfactants. ASOs can be either encapsulated within the lipid core or adsorbed onto the surface of the nanoparticle. Advantages: SLNs offer good stability, low toxicity, and controlled release profiles. The solid lipid matrix provides protection against enzymatic degradation, and the use of biodegradable lipids improves biocompatibility.
Polymer-Based Nanocarriers
Polymeric nanocarriers are versatile delivery systems that can be engineered to provide controlled release, targeted delivery, and enhanced protection against nucleases.
Polymeric Micelles Structure: Polymeric micelles consist of amphiphilic block copolymers that self-assemble into core-shell structures in aqueous environments. The hydrophobic core encapsulates the ASO, while the hydrophilic shell provides stability in biological fluids. Functionalization: The surface of polymeric micelles can be functionalized with targeting ligands, such as peptides, antibodies, or small molecules, to promote cell-specific delivery. For example, PEGylated micelles functionalized with targeting ligands can deliver ASOs specifically to tumor cells or hepatocytes.
Polyethyleneimine (PEI)-Based Nanoparticles Mechanism: PEI is a cationic polymer that forms complexes with ASOs through electrostatic interactions. PEI-based nanoparticles facilitate endosomal escape through the proton sponge effect, where PEI's amine groups buffer the endosomal pH, leading to osmotic swelling and endosomal rupture. Limitations: Although PEI enhances ASO delivery and endosomal escape, it is associated with cytotoxicity due to its strong cationic charge. Modified or biodegradable versions of PEI (e.g., low-molecular-weight PEI) are often used to reduce toxicity.
Dendrimers Structure: Dendrimers are highly branched, tree-like polymers with a central core and multiple layers of branching units. ASOs can be conjugated to or encapsulated within dendrimers, allowing for controlled release. Advantages: Dendrimers offer high loading capacity and tunable surface chemistry, which can be used to introduce targeting ligands or PEGylation for enhanced stability and cell-specific delivery.
Inorganic Nanocarriers
Inorganic nanocarriers, such as gold nanoparticles and mesoporous silica nanoparticles, provide a stable platform for ASO delivery with unique optical and physical properties.
Gold Nanoparticles (AuNPs) Mechanism: AuNPs can be functionalized with ASOs through thiol linkages, allowing for high-density ASO loading on the nanoparticle surface. The unique optical properties of AuNPs also enable real-time tracking of ASO delivery via surface plasmon resonance. Advantages: AuNPs are non-toxic and offer excellent stability in biological systems. Additionally, they can be engineered for multimodal imaging and therapeutic delivery. Limitations: While gold nanoparticles improve stability and allow for high ASO loading, their clinical use can be limited by challenges in excretion and potential long-term accumulation in tissues.
Mesoporous Silica Nanoparticles (MSNs) Structure: MSNs have a porous structure with high surface area, which allows for the encapsulation of ASOs. The porous nature of MSNs enables controlled release of the ASO in response to environmental stimuli such as pH or enzymes. Advantages: MSNs can be functionalized with targeting moieties and offer tunable pore size and surface chemistry, making them versatile carriers for ASO delivery. Their rigid structure also provides protection against nucleases.
Hybrid Nanocarriers
Hybrid nanocarriers combine the benefits of different nanomaterials to optimize ASO delivery.
Lipid-Polymer Hybrid Nanoparticles: These nanocarriers combine the biocompatibility of lipid nanoparticles with the controlled release properties of polymeric nanoparticles. The lipid layer enhances cellular uptake, while the polymer core provides structural stability and controlled release of the ASO.
Inorganic-Polymer Hybrids: Inorganic nanoparticles, such as gold or silica, are coated with polymers to improve biocompatibility and extend circulation time. This hybrid approach combines the stability of inorganic nanoparticles with the functional versatility of polymers.
Targeted Delivery with Nanocarriers
One of the most significant advantages of nanocarriers is their ability to target specific tissues or cell types, enhancing the precision of ASO delivery and minimizing off-target effects.
Active Targeting with Ligands
Nanocarriers can be functionalized with targeting ligands that recognize specific receptors or antigens expressed on the surface of target cells. This approach, known as active targeting, increases the specificity of ASO delivery to diseased tissues.
Ligands for Tumor Targeting Folic Acid: Folic acid is commonly used as a targeting ligand for cancer cells that overexpress folate receptors. Nanocarriers functionalized with folic acid deliver ASOs selectively to tumor cells, reducing off-target effects in healthy tissues. Peptides: Targeting peptides, such as RGD peptides (which bind to integrins on tumor cells), can be conjugated to nanocarriers to enhance ASO delivery to tumors.
Ligands for Liver Targeting GalNAc (N-Acetylgalactosamine): GalNAc conjugation is a powerful strategy for targeting ASOs to hepatocytes via the asialoglycoprotein receptor (ASGPR). This receptor is highly expressed on the surface of liver cells, making GalNAc a highly efficient targeting ligand for liver-directed therapies. Nanocarriers functionalized with GalNAc ensure that the ASO is preferentially delivered to the liver, reducing systemic exposure.
Passive Targeting via Enhanced Permeability and Retention (EPR) Effect
Nanocarriers also leverage passive targeting mechanisms, such as the Enhanced Permeability and Retention (EPR) effect, which is particularly useful in cancer therapy.
EPR Effect: The EPR effect refers to the tendency of nanoparticles to accumulate in tumor tissues due to the leaky vasculature and poor lymphatic drainage associated with tumors. Nanocarriers, especially those larger than 10 nm, are preferentially retained in tumors, allowing for enhanced ASO accumulation in tumor tissue.
Protection and Stability of ASOs in Nanocarriers
Nanocarriers provide a protective environment for ASOs, shielding them from degradation by nucleases in the bloodstream and improving their stability during circulation.
Nuclease Protection
Nanocarriers encapsulate or complex with ASOs, preventing direct exposure to nucleases. By encapsulating ASOs within a lipid or polymeric shell, nanocarriers reduce the likelihood of nuclease-mediated degradation, thus extending the ASO's half-life.
Controlled Release: Nanocarriers can be engineered to release ASOs in a controlled manner, either through pH-responsive release (where the ASO is released in acidic environments like endosomes) or enzyme-triggered release (where specific enzymes in the target tissue degrade the carrier, releasing the ASO).
Enhanced Pharmacokinetics
Nanocarriers improve the pharmacokinetics of ASOs by prolonging their circulation time and reducing rapid renal clearance. PEGylation of nanocarriers, for example, creates a hydrophilic shield around the nanoparticle, reducing opsonization and clearance by the reticuloendothelial system (RES).
Reduced Renal Clearance: Free ASOs are typically small enough to be rapidly cleared by the kidneys. Nanocarriers increase the overall size of the ASO complex, reducing renal clearance and increasing the ASO's exposure time to the target tissue.
Endosomal Escape Mechanisms
After cellular uptake, nanocarrier-bound ASOs are typically trapped in endosomes. Endosomal escape is a critical step to ensure that ASOs reach their target mRNA in the cytoplasm or nucleus.
Proton Sponge Effect: Cationic polymers, such as PEI, facilitate endosomal escape through the proton sponge effect. When these polymers are internalized into the acidic endosomal environment, they buffer protons, leading to an influx of ions and water. This causes the endosome to swell and rupture, releasing the ASO into the cytoplasm.
pH-Sensitive Lipids: Some lipid-based nanocarriers are designed to destabilize in response to acidic pH, which is characteristic of endosomal compartments. As the pH drops, the ionizable lipids become protonated, disrupting the endosomal membrane and allowing the ASO to escape.
In Vivo Biodistribution and Efficacy
Nanocarriers improve the biodistribution of ASOs by targeting specific tissues and enhancing their accumulation in diseased areas. In vivo studies measure how efficiently nanocarrier-ASO complexes are delivered to target tissues, their pharmacokinetics, and their therapeutic efficacy.
Tracking and Imaging: Nanocarriers can be labeled with fluorescent or radioactive tags to track their distribution in vivo. Techniques such as fluorescence imaging or positron emission tomography (PET) allow researchers to visualize how nanocarrier-ASO complexes are distributed across tissues and how efficiently they reach the intended target.
Pharmacokinetics: Studies measure how long nanocarrier-bound ASOs remain in circulation, their clearance rate, and their tissue distribution profiles. This information is critical for determining dosing regimens and ensuring that the ASO reaches its intended target at therapeutic concentrations.
Conclusion
Nanocarrier integration has become a critical advancement in the field of antisense oligonucleotide (ASO) therapeutics, addressing many of the delivery challenges that have historically limited the clinical efficacy of ASOs. By enhancing ASO stability, facilitating cellular uptake, and providing targeted delivery to specific tissues, nanocarriers have significantly improved the pharmacokinetic properties and therapeutic outcomes of ASO-based treatments. The ability to encapsulate or complex ASOs within lipid-based, polymer-based, inorganic, or hybrid nanocarriers provides protection from nuclease degradation while ensuring that ASOs are delivered intact to their intended intracellular targets.
Lipid nanoparticles (LNPs), cationic liposomes, polymeric micelles, and dendrimers are among the versatile nanocarrier systems that offer tunable release profiles, efficient transfection, and the ability to escape endosomal entrapment. This endosomal escape is crucial, as it allows ASOs to bypass the endosomal-lysosomal degradation pathway and reach the cytoplasm or nucleus, where they can modulate gene expression effectively. Nanocarriers also enhance ASO biodistribution by leveraging both passive mechanisms, like the Enhanced Permeability and Retention (EPR) effect, and active targeting through ligand-functionalized delivery systems. These targeting strategies allow for precise ASO delivery to specific tissues, such as tumor cells or hepatocytes, reducing off-target effects and minimizing systemic toxicity.
Furthermore, nanocarriers improve the pharmacokinetics of ASOs, extending their half-life in circulation and reducing rapid clearance by the kidneys. Surface modifications like PEGylation increase the circulation time of ASO-nanocarrier complexes and reduce immune activation, which is especially important for reducing the frequency of dosing and improving therapeutic efficacy.
In vivo studies have demonstrated that nanocarriers greatly improve the accumulation of ASOs in target tissues, allowing for more effective gene modulation and therapeutic outcomes. By enhancing the stability, specificity, and efficiency of ASO delivery, nanocarriers have revolutionized the potential of ASO therapeutics, enabling more precise and powerful treatments for a wide range of diseases, including genetic disorders, cancers, and metabolic diseases.
In conclusion, the integration of nanocarriers into ASO delivery systems represents a major leap forward in oligonucleotide therapeutics. Through their ability to improve delivery efficiency, ensure targeted and controlled release, and extend the pharmacokinetic profile of ASOs, nanocarriers are unlocking new possibilities for personalized medicine and expanding the therapeutic landscape for gene modulation therapies. Continued innovation in nanocarrier design and targeting strategies will likely further enhance the clinical success of ASO therapies, offering more effective treatments for patients with unmet medical needs.
The next article will be part 7. Optimization of ASOs and Delivery Systems, stay tuned..