(CAR) T Cells (Chimeric Antigen Receptor Cells) Biology, Engineering, and Therapeutic Applications
Chimeric antigen receptor (CAR) T-cell therapy is a revolutionary form of cancer immunotherapy in which a patient’s own T lymphocytes are genetically reprogrammed to target tumor cells.
Chimeric antigen receptor (CAR) T-cell therapy is a revolutionary form of cancer immunotherapy in which a patient’s own T lymphocytes are genetically reprogrammed to target tumor cells. Unlike natural T cells that require peptide antigen presentation on MHC molecules, CAR-T cells recognize surface antigens directly, combining the specificity of an antibody with the killing function of a T cell. This technology has achieved remarkable clinical success in refractory blood cancers as of 2022, six CAR-T cell products have been approved by the FDA for hematological malignancies. CAR-T therapy has induced high remission rates in diseases like B-cell acute lymphoblastic leukemia (ALL) and large B-cell lymphomas that were once nearly untreatable. However, despite these breakthroughs, CAR-T cells face significant challenges, including life-threatening toxicities and limited efficacy in solid tumors. Ongoing research in CAR biology, cell engineering, and manufacturing is driving next-generation improvements to broaden their therapeutic reach. In this article, we provide a detailed review of CAR-T cell biology, design and generations, mechanisms of action, manufacturing processes, genetic engineering strategies, stem cell-derived CAR-T platforms, expansion techniques, current clinical applications, and emerging innovations in the field.
CAR-T Cell Biology
T Cell Function and CAR Design: T cells are central effectors of adaptive immunity, capable of recognizing peptide antigens via the T-cell receptor (TCR) and destroying infected or malignant cells. CAR-T cells leverage this killing machinery but replace the TCR’s MHC-restricted recognition with a synthetic receptor that directly binds a tumor-associated antigen. A CAR is a modular fusion protein with several distinct domains:
Antigen-Binding Domain (scFv): The extracellular tip of the CAR is a single-chain variable fragment (scFv) derived from an antibody, typically composed of linked variable heavy and light chain regions. This scFv dictates the CAR’s specificity by binding to a target antigen on the tumor cell surface. Because it recognizes intact cell-surface molecules (proteins, carbohydrates, glycolipids) rather than peptides, CAR antigen recognition is MHC-independent, allowing T cells to target cells that evade immune detection by downregulating MHC. The choice of scFv also influences CAR binding affinity and off-target recognition.
Spacer/Hinge Region: A flexible spacer connects the scFv to the transmembrane domain. Often derived from IgG or CD8 sequences, the hinge provides distance and flexibility, which can be critical for allowing the scFv to access the target epitope and form an immune synapse. Spacer length and composition can markedly affect CAR function and signaling, as it influences the spacing between the T cell and target cell membranes during engagement.
Transmembrane Domain: This hydrophobic α-helical segment anchors the CAR in the T-cell membrane and links the external recognition domains to the intracellular signaling domains. Common transmembrane motifs come from CD3ζ, CD28, or CD8. The transmembrane domain contributes to CAR stability and surface expression, and it can influence how CAR molecules cluster upon antigen binding.
Co-Stimulatory Domain: Most CARs beyond the first generation include one or more co-stimulatory signaling domains in the intracellular portion. These are derived from T cell co-stimulatory receptors such as CD28, 4-1BB (CD137), OX40 (CD134), or others. The co-stimulatory module enhances T-cell proliferation, survival, and cytokine production upon CAR engagement. For example, a CD28 endodomain drives a faster, effector-memory T cell response, whereas 4-1BB yields a slower expansion but can promote a long-lived memory phenotype. The inclusion of co-stimulatory domains was a pivotal advance that significantly improved CAR-T cell potency and persistence in vivo.
Signaling Domain (Activation Domain): The CAR’s cytoplasmic tail always contains an activating signaling module, typically the CD3ζ chain of the TCR complex, which has immunoreceptor tyrosine-based activation motifs (ITAMs). This domain initiates T-cell activation when the CAR binds antigen, triggering phosphorylation cascades analogous to natural TCR signaling. Some first-generation CARs used the FcεRI-γ chain as an alternative activator, but CD3ζ is most common. The CD3ζ ITAMs provide the primary T-cell activation signal (Signal 1), which, together with co-stimulation (Signal 2), leads to full T-cell activation.
Generations of CARs: CAR-T cell technology has rapidly evolved through multiple “generations,” defined by which signaling domains are present in the CAR’s endodomain.. Early CAR designs provided T-cell activation alone, whereas newer generations incorporate additional signals to enhance efficacy or safety:
First Generation: Contain a single intracellular signaling module, typically CD3ζ with its ITAM motifs, and no co-stimulatory domain. These early CAR-T cells could kill target cells but showed limited expansion and persistence without supplemental signals.
Second Generation: Add one co-stimulatory domain (such as CD28 or 4-1BB) alongside CD3ζ. This provides Signal 2 upon antigen engagement, leading to greater T cell proliferation, cytokine secretion, and in vivo persistence compared to first-generation CARs. Most of the successful CAR-T products (e.g. Kymriah and Yescarta) are second-generation, using either 4-1BB or CD28 co-stimulatory endodomains.
Third Generation: Combine two co-stimulatory domains (e.g. CD28 and 4-1BB in series) in addition to CD3ζ. The rationale is to synergize benefits of multiple co-stim signals, potentially further increasing T-cell potency. Third-gen CARs have shown strong activity in preclinical models, but in clinical trials they have not yet clearly outperformed second-generation, and their complexity can increase tonic signaling (activation in absence of antigen).
Fourth Generation: Also known as TRUCKs (“T cells redirected for universal cytokine-mediated killing”), these CAR-T cells are engineered to secrete a transgenic cytokine or other factor upon activation. A common design is a second-generation CAR combined with an NFAT-responsive IL-12 gene, so that when the CAR engages tumor antigen, the T cell delivers an “armored” punch of IL-12 in the tumor microenvironment. IL-12 can activate surrounding immune cells (like macrophages and NK cells), enhance T-cell cytotoxicity (increasing IFN-γ, perforin, granzyme) and counteract regulatory T cells. Other armored CAR-T cells have been made to secrete IL-15 or IL-18, or express factors like CD40L or dominant-negative TGF-β receptors, to overcome tumor immunosuppression. For example, CAR-T cells secreting IL-18 reshaped the tumor immune milieu in solid tumor models, increasing infiltration by M1 macrophages and NK cells while decreasing suppressive Tregs and M2 macrophages.
Fifth Generation: These are “signal 3” CARs that integrate cytokine receptor signaling into the CAR itself. Typically built on a second-generation backbone, they include a truncated cytokine receptor endodomain (such as IL-2Rβ with a STAT3/5 recruitment motif) fused to the CARf. Upon antigen engagement, the CAR provides not only TCR-like activation and co-stimulation, but also triggers JAK-STAT pathways as if the T cell received a cytokine growth signal. The prototype 5th-gen design attaches an IL-2Rβ chain segment plus a STAT-binding domain to a CD28ζ CAR, so that NFAT activation (from CD3ζ) induces IL-2 production which then engages the chimeric IL-2Rβ, further promoting T-cell proliferation and persistence. Fifth-gen CARs aim to maximize T-cell expansion and survival intrinsically, without the need for external cytokine support. These designs are currently in preclinical and early clinical exploration.
Target Antigens: The choice of target antigen is a crucial determinant of CAR-T safety and efficacy. An ideal target is highly expressed on cancer cells but absent on essential normal cells. The most successful CAR target to date is CD19, a B-lineage surface protein expressed on virtually all B-cell malignancies (ALL, NHL, CLL) but not on most healthy tissues besides B cells. Anti-CD19 CAR-T cells eradicate both normal and malignant B cells, causing B-cell aplasia , a condition manageable with immunoglobulin replacement. This validated that on-target/off-tumor effects can be acceptable if the affected normal cells are non-essential. Other approved targets in hematologic cancers include B-cell maturation antigen (BCMA) on plasma cells, targeted by CAR-T therapy for multiple myeloma, and CD20 and CD22 (under investigation in ALL and lymphoma). In contrast, myeloid antigens like CD33 or CD123 in acute myeloid leukemia are shared with normal hematopoietic stem/progenitor cells, so CAR-T cells against them risk ablating normal bone marrow, a potentially lethal toxicity
. Solid tumor targets (e.g. HER2, EGFRvIII, GD2, mesothelin, IL13Rα2, CAIX) have been tested in clinical trials, but none are entirely tumor-specific. Low levels of these antigens on normal tissues have led to serious “on-target, off-tumor” toxicities in some cases (for example, CAR-T cells against carbonic anhydrase IX in renal cell carcinoma caused liver damage by attacking CAIX on bile duct epithelium). Furthermore, solid tumor antigens can be heterogeneously expressed; antigen-negative tumor cells can escape CAR-T killing. The difficulty of finding truly tumor-exclusive antigens remains a major hurdle, especially outside of the B-cell lineage. Nonetheless, as we will discuss, new strategies like dual-targeted CARs and controllable “switches” are being developed to mitigate these issues.
Mechanism of Action of CAR-T Cells
CAR-T cells eliminate cancer cells through a multi-step process: antigen recognition, T-cell activation, formation of an immune synapse, and targeted tumor cell killing via multiple effector mechanisms. While CAR signaling bypasses the need for antigen processing and MHC presentation, the downstream events share features with natural T-cell responses.
Antigen Recognition and Immune Synapse: When a CAR-T cell encounters a cell expressing its target antigen, the scFv on the CAR binds to the antigen on the tumor cell surface (e.g. CD19 on a leukemia cell). CAR binding causes receptor clustering and triggers the phosphorylation of CD3ζ ITAMs by Lck kinase, initiating the T-cell activation cascade A CAR-mediated immunological synapse forms at the T cell tumor cell interface, though this synapse is often more “disorganized” than the well-orchestrated bull’s-eye synapse of a native TCR. CAR clusters tend to be larger and more irregular, and they may recruit slightly different accessory proteins. Nonetheless, the CAR synapse brings the T cell and target cell into close contact and orients the T cell’s secretory apparatus toward the target. Within seconds of engagement, intracellular calcium flux and cytoskeletal reorganization occur in the CAR-T cell, leading to polarization of lytic granules toward the synapse.
T-Cell Activation and Signaling: Ligation of the CAR delivers an activation signal equivalent to TCR engagement (Signal 1) and, in second-generation designs, a co-stimulatory signal (Signal 2) from the endodomain. Together these signals trigger full T-cell activation: phosphorylation of ZAP70 and downstream kinases, activation of transcription factors (NFAT, AP-1, NF-κB), and induction of genes for proliferation and effector functions. The CAR’s co-stimulatory domain modulates this response; for example, 4-1BB-based CARs activate a TNF receptor signaling pathway that can enhance oxidative metabolism and memory formation, whereas CD28-based CARs signal through PI3K/AKT for rapid IL-2 production. CAR activation also drives robust autocrine and paracrine cytokine release. The net result is that the CAR-T cell enters a robust effector state: it begins to secrete cytokines and cytotoxic molecules and may undergo rapid clonal expansion if sufficient antigen stimulation is present.
Tumor Cell Killing Mechanisms: Once activated, CAR-T cells employ multiple cytotoxic pathways to kill target cells. These mirror the mechanisms used by natural cytotoxic T lymphocytes and NK cells:
Directed Exocytosis of Lytic Granules: Upon CAR signaling, the T cell’s lytic granules (secretory vesicles) polarize toward the immune synapse and fuse with the T-cell membrane, releasing perforin and granzymes into the synaptic cleft. Perforin molecules insert into the target cell membrane and oligomerize to form pores, allowing entry of granzymes. Granzyme B and other granzymes then cleave caspase substrates inside the tumor cell, activating the apoptotic pathway and leading to target cell death. This granule-mediated cytotoxicity is the primary and most rapid means of CAR-T cell killing, often inducing target cell apoptosis within hours.
Death Receptor Pathways: CAR-T cells can also kill via Fas/FasL or TRAIL/death receptor interactions. Activated T cells upregulate Fas ligand (FasL), which binds Fas (CD95) on the target cell, and some CAR-T cells (especially fourth-gen armoring strategies) may express TRAIL, which binds DR4/DR5 on tumor cells. Engagement of these death receptors triggers the caspase-8 dependent extrinsic apoptotic pathway in the tumor cell (formation of the death-inducing signaling complex, caspase cascade activation). This mechanism is slower than granzyme killing but can be important for targets that are resistant to granzyme or when CAR-T cells interact longer-term with tumor cells.
Cytokine-Mediated Killing and Immune Activation: CAR-T cells secrete a broad array of inflammatory cytokines upon activation notably interferon-gamma (IFN-γ), tumor necrosis factor (TNF-α), and interleukin-2 (IL-2), among others. IFN-γ can directly upregulate MHC and antigen presentation on tumor cells and activate macrophages. TNF-α can induce tumor cell apoptosis and vascular disruption in the tumor. IL-2 fuels the proliferation of not only the CAR-T cell itself (autocrine signaling) but also can stimulate bystander T cells and NK cells. These cytokines also contribute to the recruitment of innate immune cells into the tumor site. In some cases, CAR-T cells have been observed to induce secondary “bystander” killing of antigen-negative tumor cells, likely through these cytokine-mediated effects and the activation of the endogenous immune system (so-called epitope spreading). However, high levels of cytokine release are also responsible for the systemic toxicity known as cytokine release syndrome (discussed later).
After delivering their lethal hit, CAR-T cells can disengage and move on to engage other target cells (a process called serial killing). Interestingly, CAR-T immune synapses tend to be less stable and dissociate faster than classical TCR synapses, which may actually facilitate serial engagement of multiple tumor cells. CAR-T cells often kill target cells more rapidly in vitro than TCR-engineered T cells targeting the same antigen. The downside is that rapid and strong stimulation can drive CAR-T cells into an exhausted state or activation-induced cell death over time, especially if antigen is abundant. Therefore, achieving a balance between potent activation and sustained memory is an ongoing challenge in CAR design.
Manufacturing CAR-T Cells
Generating CAR-T cell therapy for patients is a complex, multi-step bioprocess conducted under strict GMP (Good Manufacturing Practice) conditions. Autologous CAR-T cells are derived from the patient’s own T cells, whereas allogeneic CAR-T cells are made from healthy donors or donor-derived cell sources (including stem cells). Each approach has advantages and challenges. Autologous products avoid immunologic rejection since the cells are the patient’s own, but manufacturing is individualized, time-consuming, and can fail if the patient has inadequate T cells (e.g. from prior chemotherapy). Allogeneic “off-the-shelf” CAR-T cells made from donors or pluripotent stem cells can be prepared in bulk ahead of time and administered on-demand, improving scalability and consistency. However, because allogeneic T cells are not patient-matched, they carry the risk of graft-versus-host disease (GvHD) (the donor TCRs attacking the patient’s tissues) and can be rejected by the patient’s immune system. Genetic edits like TCR gene knockout and HLA class I alteration are being utilized to create “universal” CAR-T cells that do not cause GvHD and can better evade host immune rejection. Despite these differences, the fundamental manufacturing steps for CAR-T cells are similar.
The manufacturing process can be summarized in several key steps:
Cell Collection (Leukapheresis): Peripheral blood mononuclear cells are collected from the patient (autologous) or a healthy donor (allogeneic) by leukapheresis. During leukapheresis, blood is circulated through an apheresis machine that filters out white blood cells (including T cells) and returns the rest of the blood components to the donor. This step typically yields billions of T cells along with other leukocytes. If starting from a cryopreserved donor leukopak or a stem cell source (e.g. cord blood or induced pluripotent stem cells), this step is adapted accordingly (e.g. differentiation of iPSC into T cells, described in a later section).
T-Cell Isolation and Activation: The T cells must be purified and stimulated to proliferate. In autologous manufacturing, the leukapheresis product is often enriched for T cells by depleting other cells or using selection beads. A common method is to use magnetic beads coated with anti-CD3 and anti-CD28 antibodies to simultaneously bind, isolate, and activate T cells. The CD3 stimulus provides a TCR-like signal and CD28 provides co-stimulation, driving the T cells into proliferation. Additional signals can be provided by feeder cells (e.g. irradiated antigen-presenting cells or artificial feeder cell lines) and cytokines. Interleukin-2 (IL-2) is traditionally added to support T-cell growth. Newer protocols often include cytokines like IL-7 and IL-15 during culture to preferentially expand less-differentiated T cells; one study found that an IL-2/IL-7/IL-15 cocktail improved expansion of CAR-T cells and maintained a desirable balance of CD4^+ and CD8^+ subsets. This activation/expansion phase lasts several days, during which T cells blast and start dividing. The goal is to generate a robust population of activated T cells receptive to genetic modification. Quality control checks at this stage may include verifying T-cell count, viability, and phenotype (e.g. memory subsets).
Gene Transfer (CAR Transduction/Transfection): Next, the CAR gene is introduced into the T cells to produce CAR-T cells. The prevailing method uses viral vectors typically γ-retroviral or lentiviral vectors engineered to carry the CAR transgene. These vectors integrate the CAR gene into the T-cell genome, allowing permanent CAR expression. Viral transduction is highly efficient and has been the workhorse of clinical CAR-T manufacturing. Both retro- and lentiviral CAR-T products have been approved, though lentiviral vectors are used in most products due to their ability to transduce non-dividing cells and carry larger gene inserts. Viral gene delivery usually occurs by exposing the activated T cells to vector supernatant or vector-loaded retronectin plates for a defined period. Aside from viral methods, non-viral techniques are being explored to avoid the expense and complexity of GMP virus production. One approach is to electroporate mRNA encoding the CAR, achieving transient CAR expression for a few days enough to test therapy or give a short-lived anti-tumor burst. For instance, mRNA-electroporated CAR-T cells targeting melanoma antigens have been produced and showed the capacity to kill tumor cells in vitro. Another non-viral approach uses DNA transposon systems (like Sleeping Beauty or PiggyBac) to integrate the CAR gene into the T cell genome. Transposon-based CAR-T cells have been tested in clinical trials (including CD19 CAR-T) with encouraging results and may reduce cost, though transduction efficiency can be lower than viral methods. Regardless of the method, after gene transfer the result is a population of engineered T cells expressing the CAR on their surface. At this point, manufacturing labs often perform a transduction check (e.g. flow cytometry to measure % CAR-positive T cells) to ensure sufficient gene delivery.
Expansion and Culture: Following genetic modification, the CAR-expressing T cells are cultured and expanded to reach the therapeutic dose. Typically, CAR-T products require on the order of 10^6 10^8 CAR-T cells per kg of patient body weight (exact doses vary by product). To achieve this, cells are grown in media with cytokines (IL-2 or others) for 1 2 weeks. Expansion can be carried out in various culture systems: gas-permeable static bags, G-Rex flasks, or automated bioreactors that control temperature, pH, and perfusion. Many facilities use the CliniMACS Prodigy or similar closed-system bioreactors to minimize contamination risk while scaling up cell number. These closed, automated systems allow culture parameters to be tightly regulated and enable manufacturing that is compliant with GMP standards for cell therapy. During expansion, the CAR-T cells typically undergo a numeric expansion of 100- to 1000-fold. Culture conditions are tuned to produce a cell product with the desired phenotype for example, avoiding overly differentiated end-stage effector cells that may exhaust quickly, and instead enriching for central memory T cells which have better persistence. Throughout the expansion, quality control tests are performed (sterility tests, endotoxin tests, and flow cytometry to monitor CAR expression and T-cell subsets). If the product meets predefined release criteria (viability, purity, potency, etc.), it can proceed to patient infusion.
Harvest, Formulation, and Infusion: Once the CAR-T cells have expanded to the required dose, they are harvested and formulated for delivery. The cells are typically washed and suspended in an infusion buffer with appropriate excipients (e.g. saline with 5% albumin) and then cryopreserved in a final patient dose or prepared fresh for immediate infusion. Prior to CAR-T infusion, patients usually receive lymphodepleting chemotherapy (a regimen such as cyclophosphamide + fludarabine given a few days earlier). This transiently knocks down the patient’s own lymphocytes, creating “space” and a burst of homeostatic cytokines (like IL-7, IL-15) that support CAR-T cell engraftment and expansion. The CAR-T cells are then infused intravenously into the patient, often 2 14 days after leukapheresis depending on manufacturing speed. Within 30 minutes to a few hours after thawing (for frozen products), the CAR-T cells are delivered as a one-time IV infusion. The patient is closely monitored in the days and weeks post-infusion for adverse reactions, particularly cytokine release syndrome and neurological events. CAR-T cells will proliferate in vivo upon encountering target antigen indeed, the in vivo expansion (C_max) of CAR-T cells often correlates with anti-tumor efficacy and can reach peak levels around 1 2 weeks post-infusion in responders.
This entire vein-to-vein process typically spans 2 3 weeks for autologous products, although efforts are underway to shorten manufacturing time. Each step must be performed under sterile conditions, with rigorous quality control. For autologous therapies, the final product is unique to the patient (autologous CAR-T is considered a drug product as well as a service). For allogeneic approaches, batches of CAR-T cells from a single healthy donor or a clonal cell line could yield doses for multiple patients, greatly reducing per-patient costs if successful. Scaling up allogeneic CAR-T manufacturing will likely involve large bioreactors and cryopreservation of bulk doses.
Genetic Engineering Strategies for CAR-T Cells
At the heart of CAR-T therapy is genetic modification of T cells. A range of engineering strategies are employed to introduce CAR genes and to further optimize the T cells’ performance and safety:
Viral Vectors: The majority of clinical CAR-T cells are produced using retroviral or lentiviral vectors to stably integrate the CAR transgene into T cells. These RNA viruses are modified to be replication-incompetent carriers of the CAR construct. Retroviral vectors (often γ-retrovirus) were used in the first clinical CAR trials and yield high gene transfer efficiency into dividing T cells. Lentiviral vectors (derived from HIV-1 backbones) can transduce non-dividing and dividing cells and are now widely used; for example, the FDA-approved products tisagenlecleucel and axicabtagene ciloleucel both use lentiviral transduction. Viral delivery typically results in each T cell getting 1 2 copies of the CAR gene randomly integrated in its genome. Advantages: Very efficient transduction (often >30-50% CAR^+ cells achievable, which can be further enriched by expansion), stable long-term expression, and a well-characterized manufacturing process for clinical-grade vectors. Disadvantages: Viral vector production is expensive and time-consuming; integration is semi-random and carries a low risk of insertional mutagenesis (though to date no leukemic transformation has been clearly caused by CAR vector insertion)f. There is also a packaging limit on transgene size for most vectors (around 8 10 kb for lentivirus). Additionally, patients may develop immune responses to viral vector components or have pre-existing antibodies, but this has not been a major issue clinically for ex vivo-modified cells. Researchers are refining safer integrating vectors (self-inactivating LTRs, inclusion of insulator sequences) to mitigate genome disruption risk.
Non-Viral Gene Transfer: To sidestep the costs and regulatory complexity of viral vectors, non-viral methods are in development. One approach uses transposable elements (transposons) to integrate the CAR DNA. The Sleeping Beauty (SB) transposon system has been applied to CAR-T cells as a “plasmid-based” integration method. T cells are electroporated with a DNA transposon (carrying the CAR gene) and a transposase enzyme. The transposase cuts and pastes the CAR transposon into the T-cell genome. Clinical trials using SB-transposed CD19 CAR-T cells have shown anti-lymphoma activity, demonstrating this method can produce functional CAR-T cells. Transposon systems can accommodate larger gene inserts than viruses and are relatively inexpensive. However, gene transfer efficiency can be lower, and the integration is random like retroviruses (with a different integration site profile). Another non-viral modality is mRNA electroporation, which introduces synthetic mRNA encoding the CAR into T cells. This leads to transient CAR expression on the cell surface (usually for a few days) without altering the genome mRNA-CAR T cells have been tested in human trials for safety for instance, RNA CAR-T cells against melanoma-associated antigen 4 (MAGE-A4) or mesothelin have been infused, and they can mediate short-term tumor cell killing with greatly reduced long-term risk. The shortcoming is the transient effect: repeated infusions of mRNA CAR-T would be needed to maintain a therapeutic response, which is inconvenient and may lead to immune clearance of the T cells. Newer techniques like CRISPR-based gene integration (e.g. homology-directed repair to knock-in a CAR gene at a specific locus like TRAC) are also emerging in research, potentially combining precision with permanence.
Genome Editing for Cell Enhancement: Beyond inserting the CAR transgene, gene editing tools (TALENs, CRISPR/Cas9, zinc-finger nucleases) are used to knockout or modify specific genes in CAR-T cells. A prime example is TCR knockout in allogeneic CAR-T cells: by disrupting the TCRα constant gene (TRAC) in donor T cells, the cells can no longer mount TCR-mediated graft-versus-host attacks on the patient’s tissues. This strategy has been implemented with TALEN-edited allogeneic CD19 CAR-T cells (e.g. UCART19), which showed anti-leukemia activity without causing GvHD. Similarly, knocking out CD52 (a target of the lymphodepletion drug alemtuzumab) in donor CAR-T cells has been done so that patients can be given alemtuzumab for lymphodepletion without killing the infused CAR-T cells. Another important target is PD-1, the inhibitory checkpoint receptor that tumors exploit to suppress T cells. CRISPR was used in a first-in-human trial to knock out PD-1 in mesothelioma patients’ CAR-T cells (targeting mesothelin), to prevent exhaustion and improve function Preclinical studies have shown PD-1 disruption can enhance CAR-T cell killing of solid tumors by keeping them in a more active state. Additional genetic edits under investigation include removing HPK1 or CISH (negative regulators of TCR signaling) to boost CAR-T intrinsic activity, knocking out HLA class I or overexpressing HLA-E to evade host immune clearance of allogeneic CAR-T, and deleting genes that drive exhaustion. Multiplex CRISPR editing allows simultaneous targeting of several genes for example, a recent study used CRISPR to create CAR-T cells lacking PD-1, TCR, and one of the checkpoint ligands, all in one step. As genome editing specificity improves, we expect “smart” CAR-T cells with customized genetic enhancements to become more common.
Safety Switches: Given the potential for serious or uncontrollable immune reactions, researchers have built “kill switches” or control genes into CAR-T cells to improve safety. One widely used strategy is the inducible Caspase-9 (iCasp9) suicide switch. In this system, T cells are transduced with a gene encoding a chimeric form of caspase-9 that dimerizes and becomes active when the patient receives a small molecule drug (e.g. rimiducid). If severe toxicity occurs, the drug is administered, triggering the iCasp9 to induce apoptosis in the CAR-T cells, thus purging them from the body. This approach has been tested clinically: for instance, donor T cells modified with iCasp9 have been successfully eliminated in patients to stop graft-versus-host disease. Another strategy is to co-express a “marker” antigen on CAR-T cells that can be targeted by an existing antibody for example, engineering CAR-T cells to express a truncated human EGFR, which can be targeted by the antibody cetuximab to kill the CAR-T cells if needed. Such a marker/suicide gene was included in some early CAR-T trials to facilitate cell tracking and elimination. Additionally, the CAR itself can be made “switchable” (discussed later) or designed to have an “off switch”. An example of the latter is an “inhibitory CAR” (iCAR) concept: T cells are given a second CAR that recognizes an antigen on healthy tissue and delivers an inhibitory signal (through PD-1 or CTLA-4 endodomain) so if the CAR-T cell tries to attack cells expressing that healthy antigen, it receives a negative signal and spares them. In summary, multiple layers of control are being engineered into CAR-T cells to ensure they can be rapidly shut down or toned down if unanticipated toxicities occur. Many of these safety circuits (iCasp9, truncated EGFR, etc.) have already been implemented in clinical trials with evidence that they can abrogate CAR-T cells when triggered.
Genetic engineering continues to innovate new ways to make CAR-T cells more potent, precise, and safe. “Armored” CAR-T with added genes (e.g. cytokine secretion, dominant-negative receptors) strengthen function, while “stealth” or “universal” CAR-T with certain genes removed (TCR, HLA) can be given to any patient without immune conflict. As gene editing tools advance, we expect CAR-T cells to become living drugs that are not only specific and cytotoxic but also smart enough to modulate themselves in real-time for optimal therapeutic effect.
Stem Cell Derived CAR-T Cells
A major frontier in the field is the development of CAR-T cells from renewable stem cell sources, such as induced pluripotent stem cells (iPSCs) or hematopoietic stem cells (HSCs). The motivation is to overcome limitations of donor-dependent T-cell sources and create off-the-shelf CAR-T therapies with batch-to-batch uniformity. Pluripotent stem cells can, in principle, provide an unlimited supply of starting material for CAR-T production. For example, T cells from a healthy donor can be reprogrammed into iPSCs (induced by Yamanaka factors) which are then clonally expanded, genetically engineered with a CAR and any other desired edits, and finally differentiated back into T cells bearing the CAR. This yields a T-iPSC derived CAR-T product that is clonal, homogeneous, and extensively scalable. Such cells can be cryobanked and constitute a readily available inventory, avoiding the need to manufacture for each patient on demand.
Several breakthroughs have been reported in this area: one group generated CD19-specific CAR-T cells from T cell derived iPSC clones (expressing a 2nd-generation CD19 CAR). These iPSC-CAR-T cells displayed conventional T-cell phenotypes and successfully eliminated CD19^+ leukemia in mice. Another approach derived CAR-T cells from iPSCs reprogrammed from naïve or memory T cells, showing that the resulting CAR-T cells had polyfunctional cytotoxic activity and in vivo antitumor efficacy. Notably, iPSC technology also allows precise genetic engineering at the stem cell stage for instance, knocking out the TCR or HLA in the iPSC so that the differentiated CAR-T cells are intrinsically allogeneic-compatible. Uniformity is a key advantage: a single well-characterized iPSC clone can produce a CAR-T cell product with less donor variability in cell subset composition or fitness.
However, generating fully functional T cells from pluripotent stem cells is technically challenging. T cells have a complex developmental program that normally requires thymic selection. In the lab, differentiation protocols use co-culture with stromal cells that provide Notch signaling (like OP9 cells expressing DLL1/DLL4) or artificial thymic organoid systems. Despite progress, iPSC-derived T cells often have an immature or atypical phenotype for example, skewing toward γδ T cells or innate-like T cells rather than conventional αβ T cells. Some iPSC-derived CAR-T products exhibit high expression of NK markers or only moderate in vivo persistence, which may limit their effectiveness. Additionally, the reprogramming and differentiation process is time-consuming (several weeks to months) and currently low-yield many iPSC lines fail to robustly produce T cells, and the differentiation efficiency can be low. There is also a safety consideration: any residual pluripotent cells in the final product could theoretically form teratomas, so complete maturation to T cells and absence of undifferentiated cells must be assured.
Aside from iPSCs, researchers are exploring CAR-T derivation from hematopoietic stem/progenitor cells (HSPCs), such as cord blood CD34^+ cells. HSPCs can be transduced with a CAR and then cultured on artificial thymic stroma to produce CAR-expressing T cells. This approach essentially recapitulates T-cell development ex vivo. A proof-of-concept study showed that gene-modified cord blood progenitors could yield CAR-T cells, but maintaining control over lineage commitment (to get mostly T cells and not NK/myeloid cells) is a challenge. Recent advancements, like thymic organoid cultures or notch ligand-presenting hydrogel systems, are making it feasible to derive T cells from HSCs more efficiently. HSC-derived CAR-T cells would have similar advantages to iPSC-derived ones in being off-the-shelf and possibly having a youthful “reset” telomere profile.
An intriguing product of stem cell differentiation are allogeneic CAR-T cell banks where one healthy donor’s cells could treat many patients. For example, an iPSC line from a donor homozygous for certain HLA types could be used to treat patients sharing those HLAs with minimal immune rejection. Another concept is making universal donor iPSCs by removing HLA and overexpressing immune cloaking molecules so that the derived CAR-T cells are not rejected by recipients essentially creating a “universal” CAR-T cell line.
In summary, stem cell derived CAR-T cells represent a promising platform to manufacture cell therapies at scale. The benefits include an inexhaustible cell source, the ability to do extensive genetic engineering at the single-cell stage (ensuring every CAR-T cell in the product has the edits), and improved product consistency. The challenges to overcome are achieving complete and efficient T-cell differentiation, ensuring full functionality of the resulting T cells, and meeting safety standards (no residual pluripotent cells, no abnormal mutations from reprogramming). Ongoing research is rapidly improving these processes. If successful, iPSC-derived CAR-T cells could dramatically reduce costs and expand patient access, transforming CAR-T therapy from a bespoke treatment into an off-the-shelf drug.
Culturing and Expansion Techniques
Effective expansion of CAR-T cells ex vivo is critical to obtaining sufficient cell numbers and the right phenotype for therapy. The culture methods and conditions during manufacturing can influence CAR-T cell potency, memory differentiation, and exhaustion status. Key considerations include the mode of T-cell activation, the type of culture system (static vs dynamic/bioreactor), the use of feeder cells, and the cytokine milieu.
Activation and Feeder Systems: As mentioned, the standard activation method uses anti-CD3/anti-CD28 antibody-coated beads to polyclonally stimulate the T cells. This mimics signals from antigen-presenting cells and causes T cells to proliferate. Some protocols also include feeder cells for example, irradiated PBMCs or artificial APC lines (like K562 cells engineered to express CD19 or costimulatory ligands) to provide additional growth stimulation and sustain expansion. Feeder cells can improve expansion and help maintain naive/memory phenotypes, but they introduce complexity and potential variability. Many clinical manufacturing processes have moved away from feeder cells in favor of defined bead-based systems for simplicity and consistency. Cytokine support is essential during culture: IL-2 at 50 300 IU/mL has been the workhorse growth factor promoting T-cell expansion. IL-2 drives T-cell proliferation but tends to favor effector differentiation and can promote activation-induced cell death at high concentrations. Therefore, combinations of common gamma-chain cytokines are now used to better sustain CAR-T cells. IL-7 and IL-15 together support expansion of both CD4^+ and CD8^+ T cells while preserving a central memory phenotype, as seen in studies where IL-7/15 improved CAR-T expansion and function relative to IL-2 alone. IL-21 is another cytokine sometimes added in low concentration to promote T cells with stem cell memory-like properties. Optimizing the cytokine cocktail is an area of intensive research to yield CAR-T cell products that have robust immediate cytotoxicity (from some effector cells) but also a reservoir of memory cells for long-term persistence.
Bioreactors and Closed Systems: Early CAR-T trials often used tissue culture flasks or gas-permeable bags for T-cell culture. These are manual, open systems not easily scalable. Modern manufacturing increasingly employs automated bioreactor systems that provide a controlled environment for T-cell growth. One example is the GE Wave bioreactor, a rocking disposable bag system that maintains cells in suspension with perfusion of fresh media. Another is the G-Rex (gas-permeable rapid expansion) flask, which allows dense static cultures with high oxygenation. More sophisticated are devices like the CliniMACS Prodigy, a closed-system apparatus that automates cell preparation, activation, transduction, and expansion in a single unit. Bioreactors offer better control over parameters like nutrient flow, waste removal, and cell density. The advantages of bioreactors include higher cell output, reproducibility, and reduced contamination risk (since they are closed systems). A study by Harrison et al., for instance, showed that an automated bioreactor could reproducibly expand CAR-T cells to clinical doses with less hands-on time. Bioreactors can also accommodate process monitoring e.g. measuring cell density, pH, glucose, lactate to inform feeding schedules or when to harvest. Maintaining a healthy culture (avoiding overgrowth and nutrient depletion) is important, as overly crowded cultures can lead to T-cell stress and exhaustion marker upregulation.
Scale and GMP Compliance: Manufacturing CAR-T for commercial distribution demands strict adherence to GMP regulations. This affects facility design (cleanrooms), documentation, and every reagent used. Closed-system culture methods, where the product isn’t exposed directly to the environment during processing, greatly assist GMP compliance. By using sterile tubing welders, bags, and closed bioreactors, the risk of contamination is minimized and processes can be more easily validated. Moreover, scaling out production to treat many patients might involve running multiple bioreactors in parallel (for autologous products) or a few large bioreactors (for an allogeneic product). The ability to freeze and thaw intermediate cell products (e.g. banking transduced cells before final expansion, or cryopreserving the final CAR-T dose) adds flexibility and requires demonstration that the cryopreservation does not impair cell viability or function.
Maintaining T-cell Quality: An important aspect of culture is preserving the functional “fitness” of CAR-T cells. Excessive stimulation in vitro can lead to differentiation into short-lived effector cells that produce a lot of cytokines acutely but do not persist after infusion. Researchers attempt to harvest at the optimal time often when cells have just entered logarithmic growth and before they plateau and accumulate suppressive molecules like PD-1, LAG-3, etc. Some protocols incorporate a rest phase after expansion, where CAR-T cells are placed in a lower cytokine environment to “rest” and possibly enrich for less exhausted cells. Others use metabolic interventions (like pyruvate supplementation or AKT inhibitors) during culture to favor memory cell generation. The impact of the manufacturing niche on CAR-T cell phenotype is illustrated by findings that shorter manufacturing (e.g. 5-7 days instead of 10-14) can yield a higher proportion of naive-like T cells, which may correlate with better persistence in patients. On the other hand, too-short manufacturing may not eliminate all dysfunctional cells or may yield insufficient numbers. Thus, each product has an optimized protocol balancing time, yield, and phenotype.
In summary, the culturing and expansion stage is where the CAR-T cells are “built” into a therapeutic product. Using advanced bioreactors and refined media conditions, scientists aim to generate large numbers of CAR-T cells that are potent, minimally exhausted, and therapeutically durable. This bioprocessing know-how has been crucial to making CAR-T cell therapy a reproducible and commercially viable treatment.
Clinical Applications and Trials of CAR-T Cells
CAR-T cell therapy has made the biggest impact in hematologic malignancies, particularly B-cell cancers. Multiple products have gained approval in relapsed/refractory settings, changing the standard of care for these patients. Meanwhile, efforts are underway to extend CAR-Ts to other cancers (myeloid malignancies, T-cell malignancies, solid tumors) and even non-cancer diseases (autoimmunity, viral infections), often via clinical trials. Here we review the current landscape of CAR-T clinical applications, as well as their associated toxicities and how those are managed.
Approved CAR-T Therapies (Hematologic Malignancies): As of 2023, six CAR-T cell therapies have been approved by the FDA (and EMA) all of them targeting either CD19 or BCMA, and all being autologous, second-generation CAR-T products. Below is a summary of these therapies and their indications:
Tisagenlecleucel (Kymriah®) A CD19-directed CAR-T (4-1BB/CD3ζ endodomain) from Novartis. It was the first CAR-T approved (2017) for pediatric and young adult B-ALL, after trials showed an 81% complete remission (CR) rate in refractory ALL. Kymriah is also approved for adult relapsed/refractory diffuse large B-cell lymphoma (DLBCL), achieving ~50% CR in that setting.
Axicabtagene ciloleucel (Yescarta®) A CD19-directed CAR-T (CD28/CD3ζ) from Kite/Gilead. Approved in 2017 for relapsed/refractory large B-cell lymphomas (DLBCL, primary mediastinal BCL, etc.). In the pivotal ZUMA-1 trial, Yescarta produced a 72% overall response rate (ORR) and 51% CR rate in aggressive B-NHL. It was later approved for refractory indolent follicular lymphoma as well.
Brexucabtagene autoleucel (Tecartus®) Also a CD19-directed CD28ζ CAR-T (Kite/Gilead). Approved in 2020 for relapsed/refractory mantle cell lymphoma (MCL), which is a B-cell lymphoma notoriously hard to cure. In the ZUMA-2 trial for MCL, Tecartus showed high response rates (ORR ~87%, CR ~62%) leading to approval. Tecartus has also been approved for adult relapsed B-ALL.
Lisocabtagene maraleucel (Breyanzi®) A CD19-directed CAR-T (4-1BB/CD3ζ, with a defined 1:1 CD4:CD8 composition) from Bristol Myers Squibb. Approved in 2021 for relapsed/refractory large B-cell lymphoma after at least two prior lines of therapy. The TRANSCEND trial reported a 73% ORR and 53% CR rate with Breyanzi in refractory B-NHL. Breyanzi is distinctive for its manufacturing, which separates CD4 and CD8 T cells and transduces and expands them in parallel before formulating the final product.
Idecabtagene vicleucel (Abecma®) A B-cell maturation antigen (BCMA)-targeted CAR-T (4-1BB/CD3ζ) from BMS/bluebird bio. Approved in 2021 as the first CAR-T for multiple myeloma, for patients who have failed at least 4 prior therapies. In the pivotal KarMMa trial, ide-cel achieved a 72% overall response rate in triple-refractory myeloma patients, with 28% stringent complete responses. Many responding myeloma patients enjoyed prolonged remissions, although nearly all eventually relapsed, indicating the need for further improvements.
Ciltacabtagene autoleucel (Carvykti®) A BCMA-targeted CAR-T (with two BCMA-binding scFvs in one CAR, a “bispecific” design) from Janssen/Legend. Approved in 2022 for relapsed/refractory multiple myeloma after ≥4 prior lines. In the CARTITUDE-1 trial, cilta-cel showed an exceptional 98% overall response rate, including ~80% stringent CRs. Responses have also been quite durable in ongoing follow-up. Carvykti’s dual-epitope BCMA CAR may contribute to its high efficacy. Its approval came with a Risk Evaluation and Mitigation Strategy (REMS) due to risk of delayed neurotoxicities observed in a minority of patients (some developed movement/neurocognitive syndrome weeks after infusion, possibly related to off-tumor recognition in brain tissue, which is under investigation).
These approved CAR-T therapies have transformed outcomes in their respective diseases, often yielding response rates and long-term remissions far beyond what prior salvage therapies could achieve. It’s worth noting all these products are autologous; patients must have cells collected and wait ~2 3 weeks for the CAR-T to be made. Clinical trials are ongoing to move some of these (or next-generation versions) earlier in treatment (e.g. second-line DLBCL, first-line high-risk ALL) given their potency.
Solid Tumor Trials and Challenges: The success in blood cancers has not yet translated to solid tumors in a routine way. Solid tumors pose several challenges for CAR-T cells: a paucity of truly tumor-specific antigens, an immunosuppressive tumor microenvironment, physical barriers to T-cell infiltration, and potential antigen heterogeneity. Nevertheless, numerous trials have been conducted or are underway. Targets explored include GD2 (in neuroblastoma and melanoma), HER2 (osteosarcoma, breast cancer), IL13Rα2 (glioblastoma), EGFRvIII (glioblastoma), MESO (mesothelin, in mesothelioma and pancreatic cancer), CEA (colorectal cancer), GPC3 (liver cancer), PSMA (prostate cancer), and many more. The results have been mixed generally, CAR-T cells have shown safety at lower doses in solid tumor patients and occasional signs of activity (some partial responses or transient tumor shrinkage), but complete durable remissions are rare so far in solid tumors. One early “win” was in neuroblastoma (a GD2-expressing pediatric cancer), where GD2-specific CAR-T cells (plus lymphodepletion) led to long-term remissions in a small subset of patients though others had only temporary responses or none. Another promising case was a patient with multifocal glioblastoma treated with IL13Rα2 CAR-T cells into the resected tumor cavity; the patient’s tumors regressed for several months. These anecdotal successes demonstrate that CAR-T cells can traffic to and attack solid tumors, but obstacles frequently stymie them.
Major hurdles in solid tumors include: On-target, off-tumor toxicity e.g. HER2 is expressed at low levels in lung epithelium, and one patient with HER2 CAR-T infusion died of pulmonary toxicity in an early trial. Antigen heterogeneity a CAR-T may kill antigen^+ tumor cells, only for antigen^ cells to outgrow (this was seen in trials like EGFRvIII CAR for GBM, where EGFRvIII-negative clones emerged). Poor T-cell infiltration solid tumors often have an abnormal vasculature and dense stroma; CAR-T cells may have difficulty homing to and penetrating the tumor bed. Immunosuppressive microenvironment (TME): Solid tumors frequently contain suppressor cells (Tregs, myeloid-derived suppressor cells) and high local levels of inhibitory cytokines (TGF-β, IL-10) and immune checkpoint ligands (PD-L1), which can disable CAR-T cells that do arrive. For instance, CAR-T cells in solid tumors often become exhausted expressing PD-1, LAG-3, and losing effector function. Additionally, repeated exposure to antigen without full clearance (the common scenario in large solid tumors) can drive T-cell differentiation towards a terminal state.
Despite these difficulties, several strategies are being pursued in trials to improve solid tumor CAR-T efficacy. Some involve combination therapies: e.g. giving checkpoint inhibitors (like pembrolizumab) after CAR-T to block PD-1 and rejuvenate T cells or combining CAR-T cells with immune modulators or other therapies. In a notable mesothelioma trial, patients received intrapleural mesothelin CAR-T cells followed by systemic anti-PD-1 antibody; this led to tumor regressions or disease stabilization in 63% of patients, a result significantly better than CAR-T alone. Other approaches include locoregional delivery of CAR-T cells to the tumor site (e.g. CAR-T injected into the cavity after surgical resection, or into the pleural space for pleural tumors) to overcome homing issues. Some trials use lymphodepletion and cytokine support even more aggressively in solid tumors to aid CAR-T expansion. Moreover, the emerging engineering innovations (armoring CAR-T cells to resist suppression, dual-target CARs to address heterogeneity, etc., discussed in the next section) are largely aimed at making CAR-T cells more effective against solid tumors.
CAR-T for Other Hematologic Malignancies: In acute myeloid leukemia (AML), CAR-T therapy has been explored targeting CD33, CD123, FLT3, and other antigens, but on-target toxicity to normal myeloid progenitors remains a concern. One strategy is to use CAR-T as a “bridge to transplant” in AML tolerating short-term aplasia then rescuing with stem cell transplant. Clinical trials of CD33 or CLL-1 (also known as CD371) CAR-T cells in AML have reported some responses, but overall efficacy is still modest and antigen-negative relapse is common. In T-cell malignancies (like T-cell acute lymphoblastic leukemia or T-cell lymphomas), CAR-T development is complicated by fratricide (CAR-T cells targeting a T-cell antigen end up killing each other). Creative solutions such as CRISPR editing out the target antigen in the CAR-T cells themselves have enabled trials to begin. For example, an anti-CD7 CAR-T (with the CD7 gene knocked out in the T cells) has shown early success in T-ALL clearing leukemia without fratricide, since the CAR-T cells no longer express CD7 themselves. These niche applications are likely to expand as engineering solutions allow CAR-T to target any cell lineage.
CAR-T for Non-Cancer Indications: While not yet as far advanced, CAR-T cells are being investigated for severe autoimmune diseases and chronic infections. The idea in autoimmunity is to target and eliminate pathogenic B cells or other immune cells essentially an immunosuppressive CAR-T. A breakthrough was reported in 2022 for lupus: CD19 CAR-T cell therapy led to deep remission in patients with refractory systemic lupus erythematosus, by depleting the aberrant B cells driving the disease. These patients had disease remission after CAR-T and even B-cell recovery later without relapse, indicating a possible “reset” of the immune system. Trials in lupus, rheumatoid arthritis, and MS are ongoing. In HIV, CAR-T cells have been made to target HIV-infected cells (e.g. a CAR against HIV envelope protein); early studies decades ago showed safety but limited efficacy. New approaches include CAR-T cells that secrete antivirals or broadly neutralizing antibodies. There is also interest in CAR regulatory T cells for treating transplant rejection or autoimmunity, by targeting T_regs to specific tissues, but that is in very early stages.
Toxicity Management (CRS and ICANS): CAR-T cells are potent “living drugs” and can cause a unique spectrum of side effects. The two hallmark toxicities are cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), previously called CAR-T-related encephalopathy syndrome (CRES). These adverse events are class-wide effects observed with all current CAR-T products, though their incidence and severity vary.
Cytokine Release Syndrome: CRS is a systemic inflammatory response triggered by massive cytokine release from activated CAR-T cells and myeloid cells (like macrophages). It usually occurs within the first few days to 1 2 weeks after infusion, coinciding with the expansion of CAR-T cells in the patient. Typical CRS begins with high fever, malaise, anorexia, and can progress to hypotension, capillary leak with hypoxia, and multi-organ failure in severe cases. Biologically, CRS is marked by elevated IL-6, IFN-γ, IL-1, TNF, and other inflammatory cytokines in the blood. High tumor burden and rapid CAR-T proliferation predispose to severe CRS. Management of CRS has improved greatly with the advent of IL-6 blockade: the anti-IL-6 receptor antibody tocilizumab can abort CRS quickly by dampening IL-6 signaling. Tocilizumab is now an essential adjunct it’s FDA-approved to treat CAR-T induced CRS. It often reverses fever and hypotension within hours. For mild CRS (fever only), supportive care (fluids, antipyretics) may suffice. For more severe CRS (hypotension requiring vasopressors, hypoxia requiring oxygen), tocilizumab is administered, and if needed, corticosteroids (dexamethasone or methylprednisolone) are added. Steroids will directly inhibit T cells and are very effective at stopping both CRS and neurotoxicity, though there was initial concern they might curtail the anti-tumor effect. Short courses of steroids do not appear to compromise long-term CAR-T outcomes in most studies, especially if used after the cellular proliferation peak. Most CRS cases (even severe ones) are reversible with prompt treatment. However, rare cases of “refractory CRS” can occur, where IL-6 blockade and steroids fail to control the inflammation. These may progress to a hemophagocytic lymphohistiocytosis (HLH)-like picture with coagulopathy, multi-organ failure, and can be fatal. Experimental therapies like IL-1 blockade (anakinra) have shown promise in preclinical models for steroid-refractory CRS, given IL-1 is an upstream driver of cytokine cascades. Indeed, anakinra is now sometimes used off-label for severe CRS/ICANS, and trials are investigating its preventive role. Other interventions, such as inhibiting catecholamines (which can fuel CRS), are being studied as well. It’s important to note that the incidence of CRS is product- and dose-dependent. For CD19 CAR-Ts, up to 70 90% of patients experience some grade of CRS, though severe grade ≥3 CRS occurs in 10 30% depending on the product. BCMA CAR-Ts in myeloma also have high CRS rates but are often low-grade fever only. The introduction of CRS management algorithms and on-call tocilizumab has made this syndrome much less threatening than in the early days of CAR trials.
Neurotoxicity (ICANS): The second distinct toxicity is neurotoxicity, now termed ICANS. Neurotoxicity typically presents after CRS (often as CRS is resolving) but can also occur independently. Patients may develop encephalopathy: confusion, word-finding difficulty, delirium, aphasia (difficulty speaking or writing), and in severe cases seizures or coma. A classic early sign is impaired handwriting or dysgraphia, which is routinely monitored with an ICE score (a neurological assessment) in CAR-T patients. The mechanisms of ICANS are not fully understood, but endothelial activation in the CNS and blood-brain barrier leakage of cytokines (and possibly T cells) are thought to contribute. Elevated IL-6, IL-1, and IFN-γ in cerebrospinal fluid have been noted. Most neurotoxicity is reversible within days to a couple of weeks, but rare cases of fatal cerebral edema have occurred (e.g. with a CD19 CAR in ALL trials). Management of ICANS involves corticosteroids, as tocilizumab does not treat neurotoxicity (IL-6 blockade doesn’t cross the blood-brain barrier well). High-dose dexamethasone or methylprednisolone is started for any ≥ Grade 2 neurotoxicity (moderate impairment) and typically results in improvement over 1 3 days. Anti-seizure prophylaxis (e.g. levetiracetam) is often given to CAR-T patients as a preventive measure. Intrathecal chemotherapy (used in ALL) does not treat CAR-T neurotoxicity, since it’s not due to leukemia in the CNS but an inflammatory process. Emerging evidence suggests IL-1 is a key mediator in neurotoxicity; indeed, blocking IL-1 with anakinra in animal models abrogated CAR-T neurotoxicity. Clinical studies are now incorporating anakinra for severe ICANS or as prophylaxis in high-risk patients. The incidence of any neurotoxicity in CD19 CAR trials is about 20 50%, but severe grade ≥3 neurotoxicity occurs in ~10 30% depending on the product (it was higher with CD28-based CARs like Yescarta, and lower with 4-1BB-based like Kymriah). BCMA CAR-Ts also cause ICANS but at lower rates (severe neurotoxicity <10% in ide-cel and cilta-cel trials). Notably, cilta-cel had some unusual late-onset neurotoxicity (movement disorders, cranial nerve palsies) occurring 1 3 months post-CAR T, possibly related to unexpected CAR T cell targeting of basal ganglia neurons expressing a low level of BCMA or cross-reactivity; this is under investigation and highlights how little we sometimes know about low-level antigen expression in the body.
In addition to CRS and ICANS, CAR-T therapy can cause other adverse effects: B-cell aplasia (on-target in CD19 CAR-T, which is expected and managed with IVIG), prolonged cytopenias (probably from the lymphodepletion chemo and cytokine effects; some patients have low blood counts for months), and increased infection risk (due to neutropenia, hypogammaglobulinemia, and immune modulation). Rarer complications include tumor lysis syndrome if disease burden is massive, or hypersensitivity reactions to the infusion (rare since it’s cells in saline). There is also theoretical risk of insertional oncogenesis from the vector, but none has been seen in CAR trials to date however, a few patients treated with CAR-T developed therapy-related myelodysplastic syndromes years later, likely from prior chemotherapies, not the CAR itself. Recently, a concern was raised about clonal expansion of CAR-T cells with vector integration near oncogenes (in one case, a CAR-T cell clone carrying a lentivector insertion next to the TET2 gene expanded, but did not cause a leukemia). This will be monitored as more patients are followed long-term.
Overall, the management of CAR-T toxicities has improved the safety profile such that, in experienced centers, treatment-related mortality is low. Current clinical trials even explore outpatient CAR-T infusion for lower-risk patients. Each approved product comes with detailed guidelines on monitoring and managing CRS/ICANS (often requiring hospital observation for at least 7 days after infusion). With burgeoning experience, clinicians can anticipate and promptly treat these toxicities, making CAR-T therapy considerably safer than it was initially perceived.
Ongoing Trials and Pipeline Developments: The CAR-T field is extremely dynamic, with hundreds of clinical trials worldwide exploring new indications and next-generation designs. Some notable directions in the pipeline include:
Earlier Line Use: Trials are testing CAR-T cells as second-line therapy for aggressive lymphoma (e.g. ZUMA-7, BELINDA, TRANSFORM trials) where CAR-T is given instead of stem cell transplant after first relapse. Initial results (e.g. ZUMA-7 for Yescarta) showed superior outcomes to standard chemo/transplant, leading to approvals of CAR-T as a second-line in certain large B-cell lymphomas. Similar trials in multiple myeloma are evaluating CAR-T vs. standard care after 1 3 prior lines. Moving CAR-T earlier could improve efficacy (since patients are less fragile and tumors less resistant) and is likely to expand approvals.
New Antigen Targets: Many trials are targeting antigens beyond CD19 and BCMA. For B-cell malignancies, CARs to CD20 and CD22 have shown activity (particularly CD22 CAR-T induced remissions in some ALL patients who relapsed after CD19 CAR-T. For AML, CARs to CD33, CD123, FLT3, CLL-1, and others are in phase 1. For Hodgkin’s lymphoma, CAR-Ts against CD30 have been tested: early trials at Baylor showed some complete remissions without significant toxicity, and a larger trial is ongoing. In T-cell leukemias/lymphomas, CARs to CD5, CD7, and TRBC1 (T-cell receptor beta chain constant region 1, present on a subset of T cells) are in development, with gene editing to prevent fratricide. Solid tumor antigen trials are too numerous to list, but include: GD2 CARs in osteosarcoma and small cell lung cancer; mesothelin CARs in mesothelioma, pancreas, ovarian (some with local regional infusions); HER2 CARs in sarcoma and breast (with lower affinities to avoid off-tumor binding); EGFR or EGFRvIII CARs in glioma and head & neck cancer; GPC3 in hepatocellular carcinoma; CEA in colorectal; MUC1 in breast and pancreas; PSMA in prostate; ROR1 in triple-negative breast and CLL; B7-H3 in pediatric tumors, etc. Many of these are early phase and focus on safety. Results have been variable, but there are hints of efficacy for example, in neuroblastoma, GD2 CAR-T combined with checkpoint blockade showed tumor regressions in some patients. Overall, the solid tumor CAR-T pipeline is actively integrating combination strategies (oncolytic viruses, checkpoint inhibitors, lymphodepletion variations) to achieve better outcomes.
Allogeneic “Off-the-Shelf” CAR-T: Companies and academic groups are trialing CAR-T cells derived from healthy donors (with gene edits to make them universal). Notable examples: UCART19 (allogeneic CD19 CAR-T with TALEN knockout of TCR) was tested in pediatric ALL and led to some complete responses, though persistence of the cells was short (patients proceeded to transplant). Allogeneic products from Cellectis/Allogene and Precision Bio are in trials for NHL and ALL. Fate Therapeutics is testing an iPSC-derived CAR NK-cell product (FT596, targeting CD19), as NK cells have natural “off-the-shelf” use (no GvHD risk). The safety of allogeneic CAR-Ts appears favorable, and no GvHD has been reported with proper TCR removal. The main issue is limited in vivo persistence, likely due to host-vs-graft immune rejection of the foreign CAR-T cells once the patient’s immune system recovers. Approaches to mitigate this include knocking out HLA molecules on the CAR-T (and/or inserting HLA-E or CD47 “don’t eat me” signals to avoid NK cell attack). One recently reported allogeneic CAR-T (anti-BCMA with CRISPR-edited TRAC, β2-microglobulin, and PD-1 genes) showed some efficacy in myeloma, albeit with limited cell persistence. As technology improves, allogeneic CAR-Ts might offer a readily available, lower-cost alternative to autologous, or even be used as bridging therapy while waiting for autologous product manufacturing.
CAR-T for Novel Diseases: Beyond oncology, early trials for systemic lupus erythematosus (SLE) have shown that CD19 CAR-T can reset autoimmunity by eliminating autoreactive B cells (achieving drug-free remission). Trials are planned or underway for other autoimmune diseases like refractory rheumatoid arthritis and multiple sclerosis, using CD19 or CD20 CAR-T to deplete B cells that drive these conditions. CAR-T is also being considered in organ transplant e.g. anti-B cell CAR-T to desensitize HLA-incompatible kidney transplant candidates by clearing alloantibody-producing B cells. In infectious disease, a novel use is CAR-T cells that target virally infected cells: for example, virus-specific T cells modified with a CAR that targets HIV envelope or HBV-infected cells expressing viral antigens. Another intriguing application is CAR macrophages (CAR-M), wherein macrophages are engineered with CARs to phagocytose tumor cells a phase 1 of CAR-M targeting HER2 in solid tumors is ongoing, marking a new branch of “CAR therapy” beyond T cells.
The pipeline is thus very rich, and the coming years will reveal whether CAR-T cells can conquer solid tumors and other diseases as effectively as they have B-cell cancers. Many of the emerging innovations described below are being applied in these trials to enhance CAR-T cell performance.
Emerging Innovations in CAR-T Cell Therapy
The field of CAR-T is rapidly innovating to address current limitations. Scientists are refining CAR designs and cellular programming to improve safety, control, specificity, and efficacy. Here we highlight several cutting-edge strategies:
Armored CAR-T Cells: So-called “armored” CAR-T cells co-express additional transgenes to bolster their function or resistance to the tumor microenvironment. A prime example is CAR-T cells engineered to secrete pro-inflammatory cytokines like IL-12 or IL-18 upon activation (these are the 4th-generation TRUCKs discussed earlier). By delivering cytokines directly within the tumor, armored CAR-T cells can modulate the local environment for instance, IL-12-armored CARs activate macrophages and NK cells and counteract T_regs in the tumor, leading to more potent anti-tumor responses. Similarly, CAR-T cells secreting IL-18 were shown to convert the tumor milieu into an inflammatory one (increasing M1-like macrophages, NK cells, and CD8 T cells) and produced impressive tumor regressions in preclinical models. Beyond cytokines, armored CARs can express factors like dominant-negative TGF-β receptors (to make T cells immune to TGF-β-mediated suppression), secrete checkpoint blocking antibodies or ligands (e.g. secrete anti-PD-L1 antibody from the CAR-T cell itself), or overexpress co-stimulatory ligands (like CD40L or 4-1BBL) to provide supplemental stimulation to themselves or neighboring immune cells. Another form of armoring is knocking out inhibitory receptors e.g. PD-1 knockout CAR-T cells behave similarly to CAR-T cells combined with checkpoint inhibitor, as they cannot be shut down by PD-L1 in the tumor. This has shown improved tumor control in animal models and early human tests. The goal of armored CARs is to make T cells that not only directly kill tumor cells, but also overcome the immunosuppressive defenses of tumors and recruit the broader immune system. Some armored CAR-T constructs (like IL-12-secreting CARs) have reached clinical trials for solid tumors (e.g. NCT02498912 testing an IL-12-secreting GD2 CAR in neuroblastoma). Safety will be a key watch-point, as constitutive cytokine release can raise the risk of CRS. Therefore, many designs use inducible expression (e.g. cytokine driven by an NFAT-responsive promoter that activates only when CAR signaling occurs in the tumor).
Switchable CAR-T Cells: One major innovation for improving CAR-T safety and control are “on/off switches” that allow clinicians to modulate CAR-T activity post-infusion. Switchable CAR-T cells are engineered such that they require a specific small molecule or protein adapter to function. In the absence of the “switch”, the CAR-Ts are inert or less active; when the switch is administered, the CAR-Ts turn on and attack the tumorl This gives a way to dose-adjust or even fully stop the CAR-T activity if toxicities arise. Several switch designs are in development:
Small Molecule Gated CARs: These split the CAR signaling machinery into two parts (often split between two fusion proteins) that only come together in the presence of a dimerizing drug. For example, one system has a CAR that lacks an intracellular domain until a drug brings it together with a separate signaling module inside the celll. The ON-switch CAR reported by Wu et al. uses two chimeric proteins one with the scFv and an intracellular co-stim domain, the other with a separate antigen-binding domain and CD3ζ neither by itself can signal, but when a small molecule (rapamycin analog) is given, it forms a complex that brings them together and reconstitutes full CAR signaling. By controlling the timing and dose of the drug, clinicians can titrate CAR-T activity. If the drug is cleared, the CAR-T cells return to an off state. This approach is in preclinical stage, but demonstrates a feasible “remote control” over CAR-T cells.
Suicide Switches: As described earlier, inducible suicide genes like iCasp9 can be considered a binary switch off (no drug, CAR-T active) vs. on (give drug to eliminate CAR-T). This is more of a termination switch rather than a reversible modulation.
Regulated Receptors: Another concept is putting the CAR under the control of a drug-regulated transcription system (e.g. a Tet-On system where giving doxycycline turns on CAR expression). This way, CAR-T cells can be deployed and later deactivated by stopping the drug, causing CAR expression to drop. This is less direct and hasn’t seen clinical use yet.
Boolean Logic CARs: These are CAR designs where the T cell requires boolean logic conditions (AND, OR, NOT) of antigen signals to activate, which can be thought of as switches keyed to antigen presence rather than a drug. For example, AND-gate CAR-T cells have been made that require two antigens: one CAR is split so that antigen A recognition leads to expression of a second CAR against antigen B (using synNotch receptor systems), thus the T cell kills only if it encounters cells expressing both A and B. While not a drug switch, it is a form of built-in control to enhance specificity and safety by preventing activation on cells that don’t meet the full antigen criteria. AND logic CARs are being explored to limit off-tumor toxicity (e.g. require a tumor antigen AND a second antigen that’s absent on normal cells) Overall, switchable CAR systems offer a safety net: clinicians could dial down the CAR-T response if CRS is too intense (by pausing the activating drug) or shut off the therapy once the tumor is cleared to avoid long-term side effects. One prominent switchable platform in the clinic is the Calibr/Oncternal system: it uses an engineered “universal” CAR (called CLBR001) that is inert until the patient is given a special antibody (called SWI019) that targets CD19 and carries a peptide tag which the universal CAR recognizes. In a Phase 1 trial, patients with lymphoma were treated with CLBR001 cells and doses of SWI019; significant responses were observed, and because the CAR-T cells themselves are not specific until the switch antibody is given, their activity can be controlled by withholding or re-dosing the antibody. This is a fusion of the “switchable” and “universal” CAR concepts.
Universal CARs (Modular Targeting): Traditional CAR-T cells are built for one fixed antigen. Universal CAR platforms aim to create a “one-size-fits-all” CAR-T cell that can be directed to various targets by use of an intermediary molecule. These systems split antigen recognition from T-cell activation. One popular approach is the bispecific adapter model: CAR-T cells are engineered to express a receptor that binds not the tumor directly, but a common tag on a separate targeting molecule (like an antibody). For instance, a CAR that recognizes the Fc portion of any IgG could be co-administered with tumor-specific antibodies; by changing the antibody, you retarget the CAR-T cells to different antigens. However, using native Fc has drawbacks (risk of activating other immune cells, off-target binding). More refined are “tag-specific” CARs: e.g. CARs that recognize a small molecule hapten, a peptide, or a non-mammalian epitope. An example is the BioAffinity Switchable CAR (BASiC) from Liu et al., where T cells have a CAR that binds a biotin molecule; the patient is given biotinylated antibodies that attach to the tumor cells, and then CAR-T cells bind those via the biotin tag. Another is the UniCAR system using a CAR that recognizes a short peptide epitope (like E5B9 tag) and inert small adapters that fuse that peptide to an anti-tumor single-chain antibody. These adapters act as a bridge between CAR-T and tumor cell, and can be cleared rapidly if needed. Universal CAR platforms offer versatility the same CAR-T cells could be redirected to a new antigen if the tumor changes, simply by switching the adapter. They also allow antigen multiplexing by giving multiple adapters simultaneously to target heterogeneous tumors. Many modular CAR approaches are in early clinical testing. The Universal CAR from a 2019 study used a FITC-specific CAR and then used FITC-labeled tumor antibodies (an approach known as CART-Bridge or Antibody-Tagged CAR). The results showed that the CAR-T could be titrated by adjusting antibody dosing, and when the antibody cleared, the CAR-T cytotoxicity subsided, adding a layer of safety. One challenge with universal CARs is ensuring the adapter’s in vivo pharmacokinetics align with T-cell needs and that the adapter doesn’t itself trigger immune responses or toxicity. Nonetheless, this concept could streamline therapy: a single CAR-T cell product (say, targeting a dummy tag) could be manufactured and stockpiled, and then a physician chooses the targeting adapter for each patient’s tumor antigen profile akin to how bispecific T-cell engagers (BiTEs) work but with an actual T-cell drug. This could significantly reduce manufacturing costs and time.
Dual-Target and Logic-Gated CAR-T Cells: To combat antigen escape and improve tumor specificity, CAR-T cells are being engineered with more complex logic. Dual-target CAR-T means the T cell can recognize two different antigens, either through co-expression of two CARs or a single CAR that has two binding domains. There are multiple configurations:
“OR” Gate (Multi-specific): T cells express two independent CARs, and if either target is present, the T cell will activate. This can reduce antigen-negative relapse for example, a CAR-T product with both anti-CD19 and anti-CD22 CARs has been tested in ALL, so that even if tumor cells lose CD19, the CAR-T can still kill via CD22 recognition. Similarly, a combinatorial CAR targeting both HER2 and IL13Rα2 is in glioma trials to prevent escape. Another OR approach is a single tandem CAR (TanCAR) that has two scFvs in tandem on one CAR molecule; this can sometimes allow bivalent binding (increased avidity if both antigens on the same cell) or binding to either antigen. A tandem CD19/CD20 CAR has shown potent activity in preclinical lymphoma models, and a tandem CD19/CD22 CAR is in clinical tests. The OR-gate is useful when either antigen alone is sufficient to mark a malignant cell (and you want to broaden coverage). It has the drawback that off-tumor cells expressing either antigen could be targeted, so it doesn’t inherently improve safety if either antigen is on healthy cells.
“AND” Gate (Dual Requirement): Here, the CAR-T is engineered such that it will only fully activate when two separate antigens are both encountered theoretically increasing specificity to tumor cells that uniquely co-express both markers. This is often implemented with a split CAR system: e.g. CAR #1 provides a costimulatory signal but no CD3ζ, and CAR #2 provides CD3ζ but is inert without costimulation. If target cell expresses both antigens A and B, the T cell receives both signals and becomes fully activated; if a cell expresses only one, the partial signal is insufficient. Another way is synNotch receptors: a synthetic Notch receptor can be made to recognize antigen A and, when triggered, it induces the expression of a CAR against antigen B. The T cell thus first needs to see antigen A, then it will upregulate the CAR B and kill cells with B enforcing an order of operations and location-specific targeting (e.g. a CAR-T might only turn on its killing mode if it has trafficked to a tissue microenvironment expressing the first antigen). AND-gate CAR designs are being tested to spare normal tissue. For example, one study designed an AND gate for prostate cancer: CAR-T cells were made to kill only if they saw PSMA and PSCA together, reducing attack on cells that expressed one of those which individually have some normal expression.
“NOT” Gate (Inhibitory CARs): This involves giving T cells an inhibitory receptor that actively suppresses activation when it encounters a certain antigen. For instance, an inhibitory CAR (iCAR) can be made with an antigen-specific scFv fused to the intracellular domain of CTLA-4 or PD-1; if the T cell engages that antigen (presumably on a normal cell), it delivers a negative signal that overrides the activating CAR signal. A proof of concept showed T cells with a GD2 CAR (activating) and an iCAR for a normal antigen present on nerves could avoid attacking cells expressing the normal antigen. This strategy is still in early research, as the timing and strength of inhibitory signaling are critical to get right.
These logic-gated approaches aim to make CAR-T targeting more precise multi-antigen targeting to prevent tumor escape, and conditional activation to reduce off-tumor hits. They also underscore an ongoing trend: treating the T cell not just as a drug but as a programmable entity, using synthetic biology to give it decision-making circuits. While more complex to engineer, the hope is that such “smart” CAR-T cells will be safer and effective even against heterogeneous solid tumors.
Combining CAR-T with Other Therapies: Finally, an emerging paradigm is that CAR-T cells might work best as part of a combination regimen rather than a monotherapy. One obvious combination is with checkpoint inhibitors, which has already been mentioned. Administering PD-1 or PD-L1 blocking antibodies alongside CAR-T (either concurrently or shortly after infusion) can counteract T-cell exhaustion and has shown synergy in preclinical models. Clinically, this is being done in trials for example, adding pembrolizumab after CD19 CAR-T in lymphoma patients who have a partial response, to try to deepen it. Early reports indicate some cases of further tumor regression, though controlled studies are needed. Another combination is CAR-T plus kinase inhibitors or other targeted drugs: e.g. ibrutinib (a BTK inhibitor) was given with CD19 CAR-T in CLL patients to help overcome the immunosuppressive CLL environment; it appeared to improve CAR-T expansion and responses. CAR-T cells might also be combined with vaccines that stimulate antigen spreading or with oncolytic viruses that inflame the tumor and even infect cancer cells with a target that CAR-T can recognize. Radiation therapy might be used to “prime” a tumor by inducing more antigen or chemokines to attract CAR-T cells. Moreover, some CAR-T cells are being designed to secrete checkpoint inhibitors or cytokines (as discussed) which is essentially combining two therapies in one cell. The line between what is a “combination” and what is a “multifunctional CAR-T cell” is blurring.
In essence, the next generation of CAR-T cells will be more programmable, addressable, and integrated. The innovations of armored CARs, switchable controllers, universal adapters, logical gating, and adjunct therapies all converge on the goal of expanding CAR-T efficacy to new diseases while minimizing adverse effects. The ongoing deep research and clinical trials will reveal which of these strategies translate into real-world cures. Given the remarkable progress in just the first decade of CAR-T clinical experience, there is optimism that engineering solutions will continue to surmount current obstacles. CAR-T cells began by proving that we can successfully “train” the immune system to destroy cancers. Now, with sophisticated bioengineering, we are evolving this living therapy into a smarter, safer, and more powerful weapon one that may eventually tackle solid tumors, autoimmune disorders, and beyond, fulfilling the broad promise of cell therapy.
References: CAR-T cell therapy represents a fusion of cutting-edge immunology and genetic engineering, and our understanding is continually refined by preclinical studies and clinical trial results. This review has cited a selection of key peer-reviewed studies and data reports that inform the current state of the field, including foundational design principles, pivotal clinical outcomes, and recent innovations, among others. The rapid advancement of CAR-T technology exemplifies the potential of cellular therapies, and ongoing research efforts aim to unlock next-generation CAR-T cells that are more universal, controllable, and effective against the most formidable diseases. With each innovation, we move closer to a future in which engineered T cells become a mainstream modality for treating not only cancer but a host of immune-related conditions, offering durable remissions and even cures for patients who previously had few options.
References
June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379(1):64 73. doi:10.1056/NEJMra1706169
Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol. 2018;15(1):31 46. doi:10.1038/nrclinonc.2017.128
Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439 448. doi:10.1056/NEJMoa1709866
Wang X, Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics. 2016;3:16015. doi:10.1038/mto.2016.15
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi:10.1038/s41408-021-00459-5
Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19(3):185 199. doi:10.1038/s41573-019-0051-2
Hartmann J, Schüßler‐Lenz M, Bondanza A, Buchholz CJ. Clinical development of CAR T cells—challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. 2017;9(9):1183 1197. doi:10.15252/emmm.201607485
Wang Z, Wu Z, Liu Y, Han W. New development in CAR-T cell therapy. J Hematol Oncol. 2017;10(1):53. doi:10.1186/s13045-017-0423-1
Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17(3):147 167. doi:10.1038/s41571-019-0297-y
Sommermeyer D, Hill T, Shamah SM, et al. Fully human CD19-specific CAR T cells with enhanced persistence and in vivo antitumor activity. Blood. 2017;130(4):229 238. doi:10.1182/blood-2016-11-751234
Themeli M, Kloss CC, Ciriello G, et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013;31(10):928 933. doi:10.1038/nbt.2678
Townsley DM, Dumitriu B, Young NS. Bone marrow failure and CAR-T cell therapy: toxicities and management. Blood Rev. 2020;44:100675. doi:10.1016/j.blre.2020.100675
Turtle CJ, Hanafi L-A, Berger C, et al. CD19 CAR T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123 2138. doi:10.1172/JCI85309
Xu Y, Zhang M, Ramos CA, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014;123(24):3750 3759. doi:10.1182/blood-2014-01-552174
Liu X, Jiang S, Fang C, et al. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 2015;75(17):3596 3607. doi:10.1158/0008-5472.CAN-15-0159
Qin VM, D’Souza C, Neeson PJ. CAR-T therapy for solid tumors: navigating the road ahead. Front Cell Dev Biol. 2021;9:746502. doi:10.3389/fcell.2021.746502
Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16(6):372 385. doi:10.1038/s41571-019-0184-6
Dufait I, Liu Z, Rommens C, et al. Armored CAR T cells: the next step in CAR T-cell therapy. Biotechnol Adv. 2021;49:107739. doi:10.1016/j.biotechadv.2021.107739
Watanabe K, Terakura S, Martens AC, et al. Target antigen density governs the efficacy of anti CD20-CD28-CD3ζ chimeric antigen receptor modified effector CD8+ T cells. J Immunol. 2015;194(3):911 920. doi:10.4049/jimmunol.1402362
Nellan A, McCully CM, Cruz Garcia R, et al. Durable remission following CD19 CAR-T therapy in a child with refractory CNS leukemia. Blood Adv. 2018;2(23):3347 3351. doi:10.1182/bloodadvances.2018021333



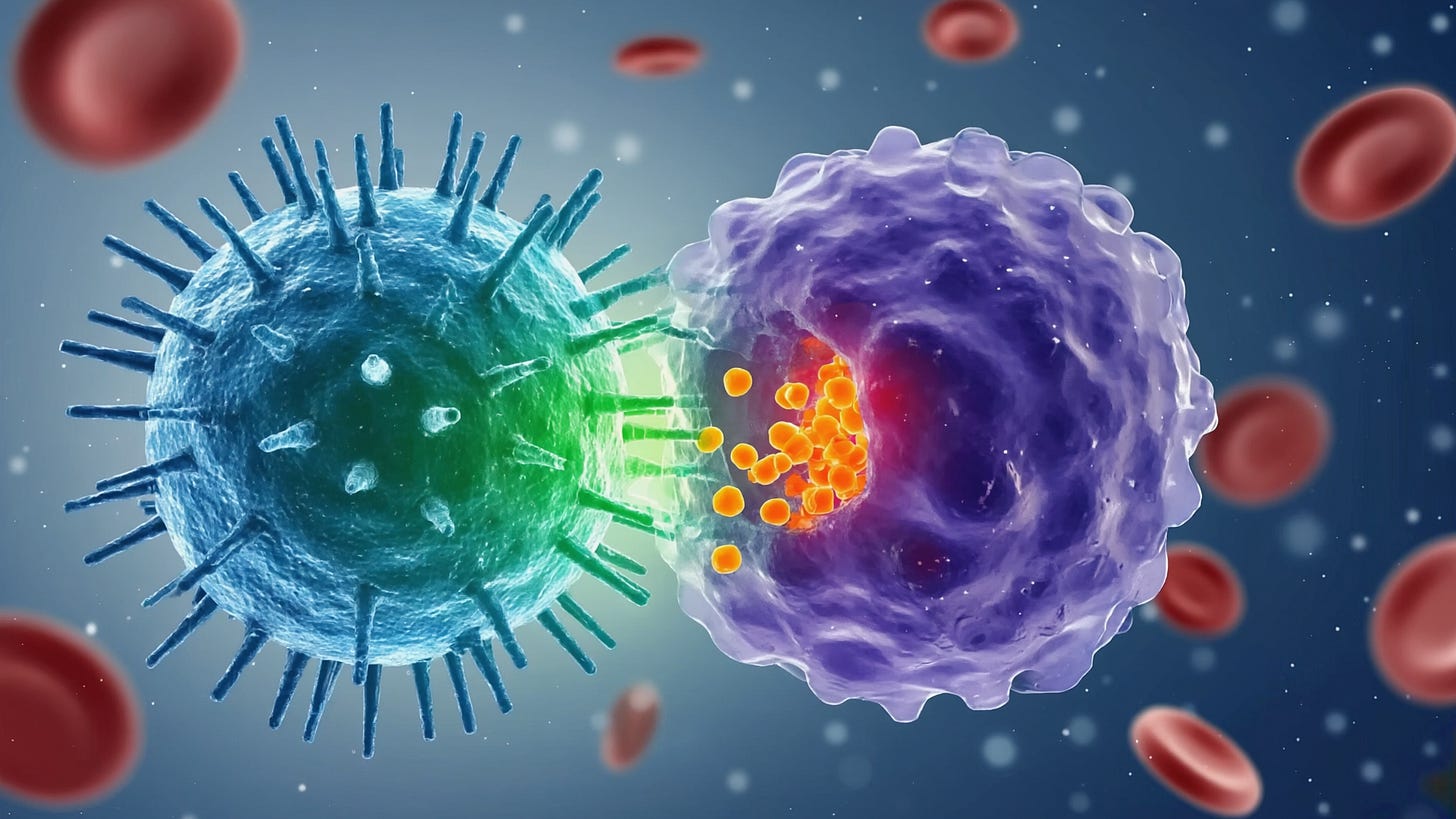
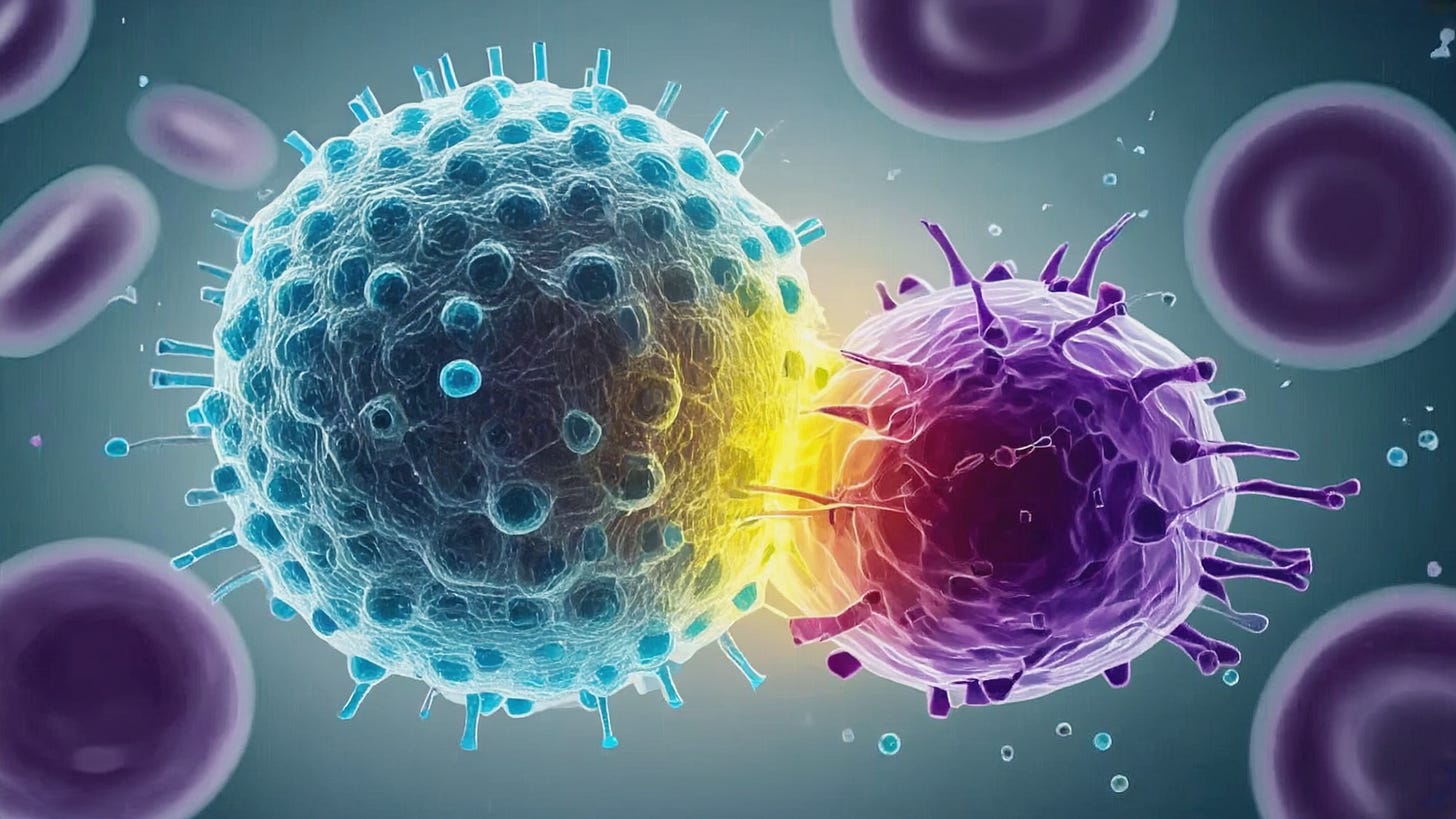
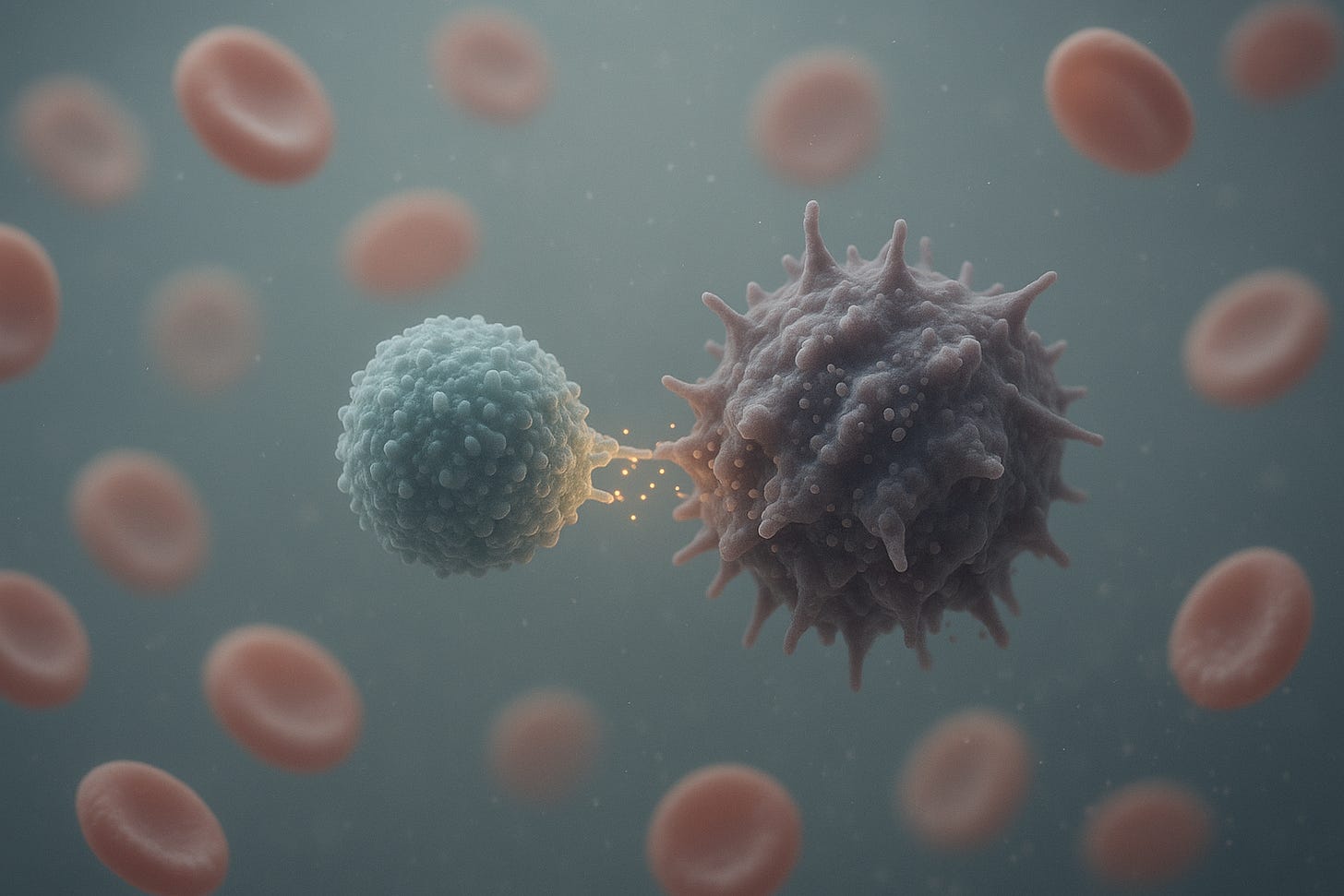
Great article, thanks