Tiny Viruses, Big Impact: The Science and Future of Phage Display
Phage display is a sophisticated and highly versatile molecular biology technique that has transformed the fields of drug discovery, structural biology, diagnostics, and enzyme engineering.
Phage display is a sophisticated and highly versatile molecular biology technique that has transformed the fields of drug discovery, structural biology, diagnostics, and enzyme engineering. Originating in the mid-1980s, this technology leverages bacteriophages—viruses that infect bacteria—to display a wide range of foreign peptides, proteins, or antibody fragments on their surfaces. This display, typically on filamentous phages like M13 or lytic phages such as T7, creates a direct genotype-phenotype link where the genetic information within the phage is directly tied to the functional protein displayed on its surface. This linkage enables the rapid screening of vast combinatorial libraries, allowing researchers to identify high-affinity binders with remarkable specificity and accelerate the development of targeted therapeutics.
The central mechanism of phage display relies on genetic manipulation of bacteriophages to incorporate foreign DNA sequences, which encode for the desired protein or peptide. These sequences are fused with genes that encode coat proteins—primarily pIII or pVIII in M13-based systems—resulting in the display of the target protein on the phage surface. M13, a non-lytic filamentous phage, extrudes from the host bacterium without killing it, allowing for continuous production and easy scaling of phage libraries. This feature makes M13 ideal for high-throughput screening applications. In contrast, T7, a lytic phage, has a more rigid capsid that allows it to tolerate harsher selection conditions. It replicates rapidly within its bacterial host, releasing a burst of phages upon lysis, which accelerates the overall screening process. The choice of phage and display protein significantly influences the properties of the library, including the sensitivity to low-affinity interactions, the valency of displayed peptides, and the overall stability of the system under stringent conditions.
Constructing a phage display library involves a complex series of genetic and biochemical steps designed to maximize diversity and optimize expression. The initial phase requires precise DNA preparation, where foreign sequences are inserted into vectors like phagemids—a hybrid of plasmid and phage DNA that allows for easier manipulation. These vectors are then introduced into a suitable bacterial host, typically E. coli, using high-efficiency transformation techniques such as electroporation. The process demands meticulous control over codon usage and translation efficiency, as foreign DNA often contains codons that are rare in E. coli, leading to expression bottlenecks. Codon optimization, ribosome binding site adjustments, and the use of synonymous codons preferred by E. coli are critical steps to ensure proper protein folding and display efficiency. Additionally, mutagenesis techniques like error-prone PCR and DNA shuffling are used to introduce random mutations, creating a diverse library that explores a broad sequence space around the target protein.
Once constructed, the library undergoes multiple rounds of selection through a process known as biopanning. This iterative technique refines the pool of phages to enrich those displaying high-affinity binders. Biopanning begins with immobilizing the target antigen on a solid support—such as microtiter plates, magnetic beads, or plastic surfaces—often utilizing methods like biotin-avidin coupling or covalent cross-linking to ensure stable and oriented antigen presentation. The phage library is then incubated with the target under carefully controlled conditions. Stringency increases with each round, involving modifications to the washing steps (e.g., using detergents like Tween-20), adjusting buffer conditions, and manipulating incubation times to favor phages with desirable kinetic properties. Nonspecific binders are gradually eliminated through negative selection steps, which involve incubating the phage library with irrelevant targets or blockers, reducing background noise and enhancing specificity.
Throughout the biopanning process, challenges such as avidity effects—where multiple low-affinity interactions create an artificially strong overall binding—and false positives due to nonspecific binding must be addressed. Phage particles, especially those utilizing the pVIII coat protein, display thousands of copies of the target peptide, potentially masking weaker but more specific interactions. To mitigate these effects, researchers often switch to monovalent systems, like pIII-based displays, where fewer copies of the protein are presented, providing a more accurate measure of true binding affinity. Additionally, techniques like negative biopanning and counter-selection are employed to filter out phages that show nonspecific or irrelevant binding. Successful candidates are amplified in E. coli through selective growth conditions, and the cycle repeats for several rounds, progressively increasing the selection stringency to isolate high-affinity clones.
After biopanning, the selected phages undergo extensive analysis to validate their binding specificity, affinity, and functional properties. Sequencing of the selected clones is a critical step, revealing if convergent evolution has occurred—a hallmark of effective selection where multiple independent clones exhibit similar sequences, indicating strong binding properties. Sanger sequencing is suitable for small-scale analysis, while next-generation sequencing (NGS) provides a comprehensive overview of the library’s diversity and the enrichment dynamics over successive rounds. Binding assays like ELISA (Enzyme-Linked Immunosorbent Assay) and SPR (Surface Plasmon Resonance) are employed to quantify the binding strength and kinetics of selected phages, offering insights into the rates of association (on-rate) and dissociation (off-rate). SPR, in particular, provides real-time kinetic data, allowing for the determination of equilibrium dissociation constants (K"D"), a critical parameter for assessing the overall binding affinity.
Advanced characterization techniques extend beyond simple binding analysis to include epitope mapping, thermodynamic profiling, and structural elucidation. Epitope mapping identifies the precise regions of the target antigen recognized by the selected binders, using methods like alanine scanning to pinpoint crucial residues. Thermodynamic characterization, through methods such as Isothermal Titration Calorimetry (ITC), assesses the enthalpic and entropic contributions to binding, revealing the stability and strength of interactions. For structural details, X-ray crystallography or Cryo-Electron Microscopy (Cryo-EM) can visualize the precise molecular interactions at an atomic level, providing insights into the binding interface and guiding further optimization.
Phage display’s utility spans several disciplines, from drug discovery to vaccine development, diagnostics, and protein engineering. In drug discovery, it plays a pivotal role in identifying monoclonal antibodies and peptide inhibitors, where libraries are tailored to specific therapeutic targets. The iterative nature of phage display facilitates affinity maturation—enhancing the binding properties of selected candidates through directed evolution. This involves sophisticated mutagenesis techniques, including error-prone PCR to introduce diversity and DNA shuffling to combine beneficial mutations. These libraries are refined through advanced selection strategies, such as focused epitope libraries that concentrate on known binding sites or regions of interest, reducing screening time and increasing efficiency.
Diagnostic applications of phage display involve developing highly specific antibodies for disease biomarkers. These antibodies serve as the foundation for ELISA, lateral flow assays, and other diagnostic platforms, enabling the rapid and accurate detection of pathogens or disease-associated proteins. Epitope mapping is crucial for diagnostics, allowing researchers to pinpoint immune-reactive regions, aiding in the design of precise detection tools. Phage display also contributes to vaccine development by identifying immunogenic epitopes that can stimulate strong and protective immune responses. This involves screening phage-displayed peptide libraries against sera from immunized animals or patients, identifying sequences that elicit neutralizing antibodies, and enhancing vaccine efficacy through multivalent displays.
Technical challenges remain a significant aspect of phage display, requiring constant innovation to overcome inherent limitations. Constructing highly diverse libraries is constrained by transformation efficiency, codon usage, and clonal competition during amplification, which can reduce effective diversity. Addressing these issues involves using high-efficiency competent cells, codon optimization tools, and pooling multiple transformations. Selection challenges, such as nonspecific binding, avidity effects, and false positives, are managed by optimizing biopanning protocols, employing stringent washing conditions, and integrating negative selection steps. Different phage display formats, including filamentous M13, robust T7, and versatile λ, each present unique obstacles related to stability, valency, and structural compatibility. Solutions like gentle elution buffers for M13, pre-amplification for T7, and hyperphage systems to enhance display efficiency exemplify the adaptations developed to optimize each platform.
Emerging alternatives, like ribosome display and mRNA display, offer complementary strengths to traditional phage systems, bypassing the need for living cells and enabling the exploration of ultra-large libraries. Ribosome display stabilizes the ribosome-mRNA-peptide complex, linking genotype and phenotype without the constraints of transformation efficiency, while mRNA display uses a puromycin linker to physically tether the translated protein to its encoding mRNA, simplifying the selection of high-affinity binders. Computational tools for in silico modeling, docking, and rational design have become indispensable, enabling precise predictions of mutations that enhance binding or stability. These simulations guide directed evolution, complementing traditional mutagenesis methods and expediting the optimization process.
High-throughput platforms, automated screening, and deep sequencing are revolutionizing the phage display landscape. Robotic systems facilitate large-scale biopanning, integrating with ELISA and SPR setups to screen thousands of candidates efficiently. Next-generation sequencing allows researchers to track the evolution of library diversity, identify dominant clones, and optimize library construction by revealing underrepresented yet valuable sequences. These technological advances are continuously pushing the boundaries of phage display, making it an ever-evolving tool with the potential to transform not only drug discovery and diagnostics but also the development of novel biomaterials, biosensors, and industrial enzymes.
The ongoing evolution of phage display underscores its adaptability and importance in modern biotechnology. This comprehensive article delves into the intricate technical details of phage display, from the core principles that govern its mechanisms to the advanced strategies that drive its continued refinement, highlighting how this transformative technology continues to reshape our understanding and manipulation of biological interactions.
The Basics: Bacteriophages and Their Anatomy
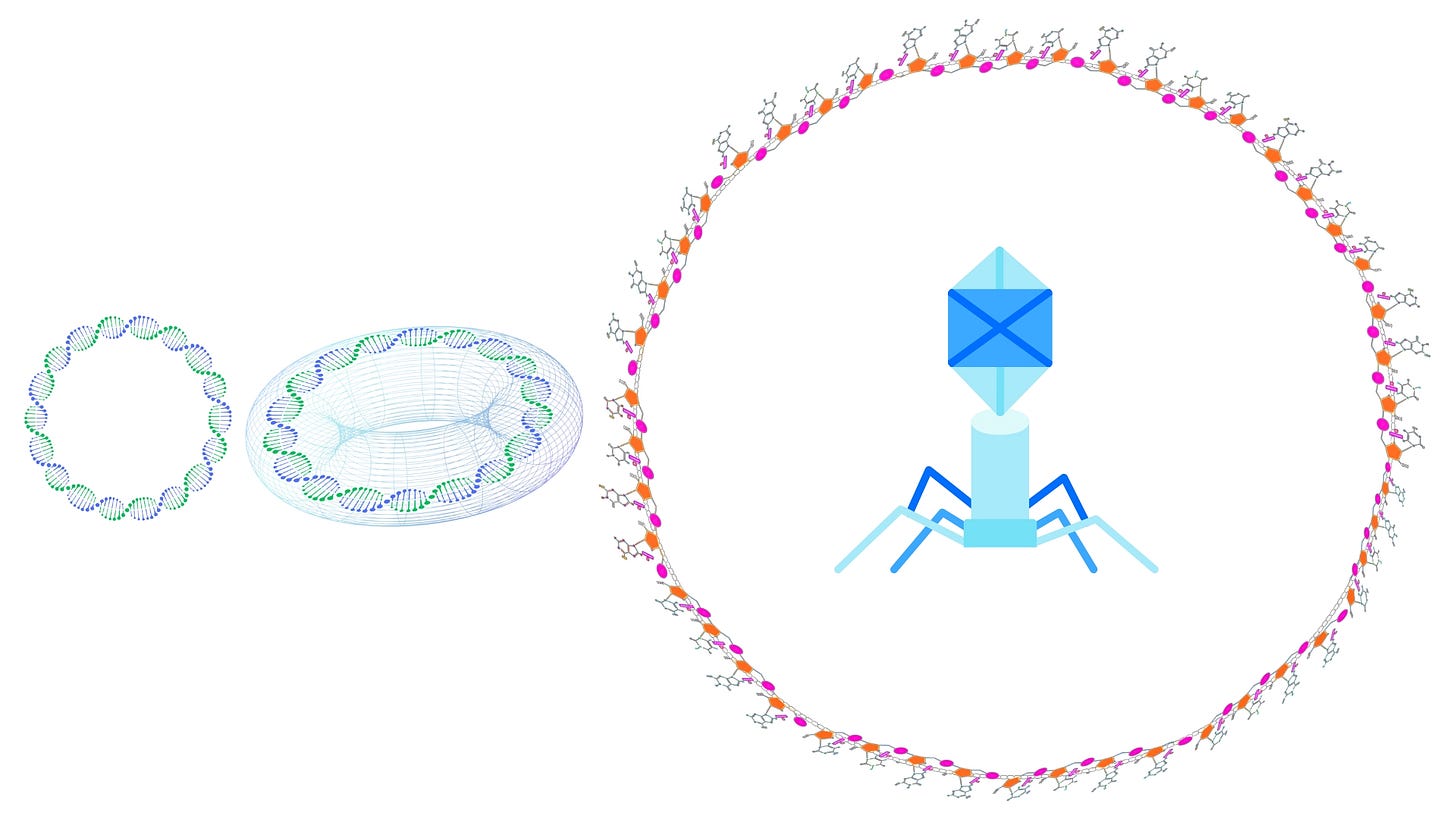
Bacteriophages, or simply phages, are viruses that specifically infect bacteria. They are integral to the technique of phage display, which utilizes the ability of these viruses to present peptides or proteins on their surface for study and manipulation. Understanding phage display technology starts with a deep dive into the structure of bacteriophages, specifically the filamentous bacteriophage M13, which is a model organism for phage display due to its unique properties.
Overview of Bacteriophage Anatomy
At a high level, bacteriophages consist of two main components:
Genetic Material: This is the DNA or RNA that the phage uses to hijack the host bacterium's machinery for replication. In the case of M13, the genome is single-stranded DNA (ssDNA).
Protein Coat (Capsid): This is a protective layer made up of multiple proteins that encase the genetic material. It also serves functional roles, such as attachment to the bacterial cell and interaction with the host's machinery.
Anatomy of Filamentous Phage M13
M13 is a filamentous bacteriophage, meaning it has a long, flexible rod-like shape, unlike other bacteriophages that have icosahedral (spherical) or complex structures. Here's a breakdown of its structure:
Capsid Proteins
M13's structure is dominated by a series of proteins that form the external shell, with each protein playing specific roles:
pVIII (Major Coat Protein):
Function: The pVIII protein is the most abundant, making up the primary coat of the phage. Approximately 2,700 copies of pVIII surround the single-stranded DNA, forming the bulk of the cylindrical capsid.
Structure: It’s a small protein, about 50 amino acids long, with a helical conformation. The arrangement of these proteins creates a helical tube, which can stretch to accommodate longer or shorter genomes.
Role in Phage Display: The pVIII protein can be modified to display small peptides, but it has limited capacity for larger proteins, as excessive modifications may affect the phage's stability or infectivity.
pIII (Minor Coat Protein):
Function: The pIII protein is essential for the infection process, responsible for attaching the phage to the bacterial host. M13 carries five copies of pIII located at one end of the phage, acting as the "business end" during the infection of E. coli cells.
Role in Phage Display: This protein is often modified to display larger proteins or peptides, as its position at the end of the phage minimizes any detrimental effects on phage integrity. Only a few copies of the modified pIII are present, reducing the impact of avidity effects (explained below).
pVI, pVII, and pIX (Minor Coat Proteins):
These proteins play supporting roles. pVI is closely associated with pIII and aids in the infection process. pVII and pIX are located at the opposite end of the phage, anchoring the single-stranded DNA into the capsid.
pVII and pIX in Phage Display: Though not commonly used, some advanced phage display systems utilize pVII and pIX for specialized applications, such as displaying complex antibody fragments.
Genetic Material
M13's genome is a circular, single-stranded DNA molecule. This genome encodes all the necessary proteins for replication, assembly, and infection of the host bacterium. The circular nature of the genome makes it highly efficient for rolling-circle replication, a process critical for producing many copies of the phage in an infected cell.
Infection Mechanism of M13 Bacteriophage
The infection process of M13 involves several steps, each facilitated by different proteins encoded in the phage’s genome:
Attachment to Host:
M13 specifically infects E. coli bacteria that have the F-pilus, a hair-like appendage that bacteria use for genetic exchange. The pIII protein of the phage binds to this structure, initiating the infection process.
This interaction between the F-pilus and pIII protein is the first critical step for infection, relying heavily on the precise conformation of pIII to attach successfully.
Genome Entry:
Once attached, pIII interacts with another bacterial protein, TolA, embedded in the bacterial membrane. This interaction facilitates the transfer of the phage’s single-stranded DNA into the bacterial cytoplasm.
The ssDNA then forms a double-stranded replicative form (RF) inside the bacterium, which acts as a template for further replication and production of phage proteins.
Phage Assembly and Release:
M13 does not lyse (break open) the host cell. Instead, it continuously extrudes new phage particles through the cell membrane. This property makes M13 ideal for phage display, as it allows for the sustained production of modified phages without killing the host.
Genotype-Phenotype Link in Phage Display
A key advantage of using phages like M13 is the genotype-phenotype link—the direct connection between the genetic material inside the phage and the protein displayed on its surface. This relationship is crucial for the affinity selection process:
Genotype: The DNA inside the phage encodes the protein being displayed.
Phenotype: The protein displayed on the capsid (e.g., an antibody fragment) can interact with specific targets.
Affinity Selection: If a displayed protein binds to a target (like an antigen), that specific phage can be isolated, and its genetic material analyzed. This allows researchers to "fish out" phages displaying desirable binding properties.
Display Systems: pIII vs. pVIII
The choice between displaying proteins on pIII or pVIII proteins involves trade-offs:
pIII-based Display:
Low Avidity: Fewer copies of the displayed protein (typically fewer than five per phage), reducing the cumulative binding strength of multiple weak interactions. This makes pIII better for selecting high-affinity interactions.
Protein Size: pIII can accommodate larger and more complex proteins because it's not as structurally restrictive as pVIII.
Applications: Used for displaying complex antibody fragments, like scFv (single-chain variable fragments) or Fab (fragment antigen-binding) regions.
pVIII-based Display:
High Avidity: A large number of displayed proteins per phage (thousands), enhancing the overall binding strength. This makes it suitable for initial broad screenings where sensitivity is more important than specificity.
Size Restriction: Due to its structural role, pVIII can only handle small peptide inserts without affecting phage integrity.
Applications: Used for displaying small peptides in high-density formats, helpful in mapping small epitopes or in high-throughput screening.
Avidity Effect Explained
Definition: Avidity refers to the cumulative strength of multiple binding interactions. While a single weak interaction might not be strong, multiple weak interactions happening simultaneously can create a strong overall effect.
Implications in Phage Display:
pVIII: High avidity due to thousands of copies of displayed peptides. It’s beneficial for finding targets with multiple weak binding sites, but it can mask low-affinity interactions.
pIII: Lower avidity, providing more reliable affinity measurements because the displayed protein interacts with the target as a single entity.
Use of Phagemids
In addition to the standard M13 genome, phage display often uses phagemids, which are hybrid vectors combining features of plasmids and phage DNA:
Phagemid Properties:
Replicate like Plasmids: They contain a bacterial origin of replication, allowing them to replicate independently in E. coli.
Package like Phages: They include a phage origin of replication (f1 Ori) that enables packaging into phage particles with the help of a helper phage.
Helper Phage Role: The helper phage provides the necessary proteins for packaging, but its replication is kept in check to ensure that the phagemid is preferentially packaged.
Bacteriophages like M13 are essentially molecular machines optimized for infecting bacteria and replicating inside them. Their modular structure—consisting of a protein coat and a simple genome—makes them ideal for phage display technology, where modifications to their proteins enable the exploration of vast libraries of peptides and proteins. Understanding the intricate anatomy of these phages provides the foundation for effective use in selecting and engineering antibodies with desired properties.
The Core Concept: Display of Foreign Proteins on the Phage Surface
The idea behind phage display is to harness the ability of bacteriophages, specifically filamentous phages like M13, to present foreign proteins or peptides on their surfaces. This involves embedding foreign DNA sequences within the phage genome so that the corresponding protein is expressed and displayed on the outer coat of the phage. Below is a deep dive into the technical aspects of this process, focusing on the core principles and mechanisms that make phage display a powerful tool for molecular biology.
How Foreign Proteins Are Displayed: Key Concepts
Phage display technology relies on manipulating the genetic code of bacteriophages to produce modified coat proteins. This process involves several critical steps:
Insertion of Foreign DNA:
Foreign DNA, encoding a protein or peptide of interest, is inserted into a specific location within the phage genome.
The DNA is typically fused with the gene that codes for a coat protein, such as pIII or pVIII, which will present the foreign protein on the phage surface.
Translating DNA into Protein:
The bacteriophage’s genetic machinery translates the modified genome, leading to the production of a fusion protein—a combination of the native coat protein and the foreign peptide/protein.
This fusion protein is then incorporated into the phage's protein coat, exposing the foreign peptide/protein on the outside surface of the phage particle.
Genotype-Phenotype Link:
The sequence of the foreign DNA inserted in the phage genome (genotype) directly correlates to the protein displayed on the phage surface (phenotype).
This allows for the physical isolation of specific phages based on their binding characteristics, followed by sequencing the DNA to determine the inserted genetic code.
Where Foreign DNA is Inserted: pIII and pVIII Proteins
The two most common coat proteins used for displaying foreign proteins are pIII and pVIII, each having unique features that suit different applications:
pIII Display
Role: The pIII protein is crucial for the infection of host cells. It is located at one end of the M13 phage, with typically five copies per phage.
Insertion: Foreign DNA is inserted within or adjacent to the gene for pIII. The result is a fusion protein where the foreign peptide is attached to pIII, often at the N-terminus.
Advantages:
Allows for the display of relatively large and complex proteins.
Reduces the avidity effect, as only a few copies of the foreign protein are displayed per phage.
Applications: Ideal for displaying full-length antibodies, larger proteins, or complex domains that require proper folding. Often used in affinity selection for high-specificity interactions.
pVIII Display
Role: The pVIII protein is the major structural component of the phage, forming the primary coat and appearing in around 2,700 copies per phage.
Insertion: DNA is typically inserted into the gene encoding pVIII, resulting in small peptides being fused to the N-terminus of the protein.
Advantages:
Can display thousands of copies of the foreign peptide, leading to high sensitivity during screening.
Suitable for displaying short peptides, epitopes, or small protein fragments.
Applications: Used for high-throughput screening, epitope mapping, and identifying small peptide sequences with desired binding properties.
Constructing Fusion Proteins
The construction of fusion proteins—where a foreign peptide or protein is linked to a coat protein—is a critical step in phage display. This construction requires precise genetic engineering to ensure the correct folding, display, and function of the fusion protein:
Fusion Protein Design
Linkers: Often, a flexible linker sequence is inserted between the coat protein and the foreign peptide. This linker:
Prevents steric hindrance (physical blocking) between the foreign protein and the phage surface.
Enhances the folding and presentation of the foreign protein.
Cleavage Sites: Sometimes, a protease cleavage site is included in the linker region, allowing the foreign protein to be separated from the phage coat if needed, which is useful in downstream applications like purification.
Codon Optimization
When inserting foreign DNA into the phage genome, the genetic code is often optimized for the host organism (typically E. coli), ensuring efficient translation and proper folding.
This involves choosing synonymous codons that are most commonly used by E. coli, which can significantly improve the expression levels of the fusion protein.
Technical Considerations: Impact on Phage Fitness
Introducing foreign proteins onto the surface of a bacteriophage can affect its fitness and stability. There are several technical challenges to ensure that the modified phage remains viable:
Phage Stability
Size Constraints: Larger insertions in the pVIII protein can destabilize the phage, causing structural defects that might affect replication and packaging.
Structural Compatibility: Foreign proteins must fold correctly to maintain the phage's structural integrity. Poor folding can lead to aggregation or improper assembly, decreasing infectivity.
Fitness Trade-Offs
Adding foreign proteins can sometimes reduce the efficiency of the infection process, particularly when pIII is heavily modified, as it plays a crucial role in host attachment.
Some phage variants might grow slower or have lower yields, impacting the overall efficiency of library screening.
Advanced Display Formats
Researchers have developed variations of the standard phage display systems to improve display efficiency, binding strength, and library diversity:
Monovalent vs. Multivalent Display
Monovalent Display: Only one or a few copies of the foreign protein are displayed per phage. This setup is used to identify high-affinity binders because it minimizes the avidity effect.
Multivalent Display: Many copies of the foreign protein are displayed on the phage. This increases sensitivity but may favor low-affinity interactions due to cumulative weak binding.
Dual-Display Systems
In some systems, two different proteins can be displayed simultaneously on the same phage. This is useful for studying protein-protein interactions or multi-epitope recognition.
Creating and Screening Phage Libraries
Once the phage is engineered to display a foreign protein, researchers create libraries containing millions to billions of unique variants. Here's how the process works:
Construction of Phage Libraries
Combinatorial Library: A diverse set of foreign DNA sequences is inserted into the phage genome, creating a vast pool of phages, each displaying a unique protein variant.
Error-Prone PCR: Mutations can be introduced deliberately into the foreign DNA during the PCR amplification step to increase diversity and explore a wider range of sequence space.
Shuffling: Genetic material from multiple sources can be shuffled to create novel combinations, mimicking natural recombination.
Affinity Selection (Biopanning)
Biopanning is the process used to screen these large libraries:
Phages are exposed to a target antigen immobilized on a surface (like a Petri dish or magnetic bead).
Only phages displaying proteins with affinity for the target will bind.
Bound phages are washed, eluted, and then amplified in bacteria.
Iterative Rounds: The selection process is repeated multiple times with increasing stringency (e.g., reducing the antigen concentration or using more stringent washing conditions) to isolate the highest-affinity binders.
Applications of Phage Display
The ability to display foreign proteins has a broad range of applications, particularly in the field of immunology and drug discovery:
Antibody Discovery: Phage display allows the selection of antibodies that bind specifically to a target molecule, making it a powerful tool for developing monoclonal antibodies for diagnostics and therapeutics.
Epitope Mapping: Short peptide libraries can be used to identify the specific regions (epitopes) of an antigen that are recognized by antibodies.
Protein-Protein Interaction Studies: Displaying protein domains enables the study of binding interactions between proteins, crucial for understanding cellular processes.
Enzyme Engineering: Displaying enzyme variants allows for the screening of catalytic properties, facilitating the discovery of enzymes with improved characteristics.
The core concept of phage display revolves around genetic manipulation and the precise control of protein expression on a phage’s surface. By understanding the anatomy of the phage, the mechanics of foreign protein display, and the impact of structural and fitness constraints, researchers can exploit this technology to screen vast libraries, study complex biological interactions, and create targeted therapeutic agents.
Construction of Phage Display Libraries
Constructing a phage display library involves creating a vast pool of bacteriophages, each displaying a unique protein or peptide variant. This is achieved through careful genetic manipulation of phage genomes or phagemids (plasmid-phage hybrids) to introduce diverse DNA sequences, which are then translated into a myriad of protein variations presented on the phage surface. Below is an in-depth exploration of the technical steps involved in constructing these libraries, from DNA design to final screening.
Designing the Library: DNA Preparation
The first step in constructing a phage display library is designing the DNA that will encode the diverse pool of proteins or peptides:
Combinatorial Diversity
The power of a phage display library lies in its diversity. Here are several key approaches to achieving combinatorial diversity:
Random Peptide Libraries:
Short peptides of random amino acid sequences (typically 7-15 residues) are encoded by synthetic DNA oligonucleotides.
Degenerate codons (like NNK or NNS, where "N" can be any nucleotide and "K" or "S" limits the third base) are used to create a diverse set of amino acid sequences. This avoids certain codon biases and unwanted stop codons.
Randomized peptide libraries are ideal for discovering short motifs or epitopes that bind specifically to a target protein.
Antibody Libraries:
Antibody libraries are constructed using the variable regions of antibodies, particularly the variable heavy (VH) and variable light (VL) chain regions. These regions determine antigen specificity.
Libraries can be based on natural antibody repertoires extracted from immune cells or synthetically designed using computational tools to optimize binding characteristics.
Error-Prone PCR and DNA Shuffling:
Error-Prone PCR introduces random mutations into the DNA, creating point mutations that lead to a wider range of protein sequences. This method is particularly useful when seeking proteins with enhanced or novel functions.
DNA Shuffling involves fragmenting multiple DNA sequences and reassembling them randomly, simulating natural recombination. This technique creates hybrids that combine beneficial mutations from different sequences.
Codon Optimization
Codon usage can significantly impact the efficiency of protein expression in E. coli (the typical host bacterium for phage display):
Synonymous Codons: These are different codons that code for the same amino acid. By choosing the most frequently used codons in E. coli, researchers can ensure optimal translation rates and protein folding.
Reduced GC Content: Lowering the GC content (percentage of guanine-cytosine base pairs) can simplify the cloning process and minimize secondary structures that may hinder replication or translation.
Cloning the DNA: Inserting into Phage or Phagemid Vectors
The selected DNA sequences must be inserted into a vector capable of displaying the target protein on a phage:
Choice of Vector
Phage Vectors: These are derived directly from bacteriophage genomes, like the M13 genome. They replicate and package the modified DNA within the viral particle.
Phagemid Vectors: Phagemids combine features of plasmids and phage DNA, making them highly versatile:
Contain a plasmid origin of replication (Ori) for stable maintenance within E. coli.
Include a phage origin (f1 Ori) that allows packaging of single-stranded DNA (ssDNA) into phage particles when co-infected with a helper phage.
Require a helper phage for packaging, which introduces the necessary proteins for phage assembly while suppressing its own DNA packaging efficiency to favor the phagemid DNA.
DNA Insertion Methods
Restriction Enzyme Cloning:
DNA sequences are inserted into the vector at restriction sites flanking the gene for a display protein (like pIII or pVIII). This requires precise digestion with restriction enzymes and ligation.
Common vectors have multiple cloning sites (MCS) that allow flexibility in choosing insertion points.
Gateway Cloning:
A site-specific recombination technique using the attP and attB recombination sites. This method allows for seamless insertion of DNA fragments, minimizing unwanted mutations.
Gateway cloning is faster and more accurate than restriction enzyme cloning, ideal for high-throughput library construction.
Gibson Assembly:
A method that uses overlapping DNA fragments and a mix of enzymes (exonuclease, polymerase, and ligase) to join them seamlessly. It allows for the insertion of complex and large DNA sequences without the need for restriction sites.
Transformation of Competent Cells and Library Assembly
Once the DNA is cloned into the phage or phagemid vector, the next step is to introduce this construct into E. coli cells, where the library will be assembled:
Preparation of Competent Cells
Competent Cells are bacterial cells that are treated to increase their ability to uptake foreign DNA. Two primary methods are used:
Chemical Competence: Cells are treated with calcium chloride (CaCl₂), making their membranes more permeable to DNA.
Electroporation: Cells are exposed to an electric pulse that temporarily disrupts the cell membrane, allowing DNA to enter. This method is more efficient and suitable for creating large libraries due to higher transformation efficiency.
Electroporation and Transformation Efficiency
DNA Purity: The DNA used for electroporation must be of high purity. Contaminants can reduce transformation efficiency.
Cell Density: The culture density (OD600) must be precisely controlled. Optimal cell density is around 0.4 OD, representing mid-log phase growth, which maximizes transformation efficiency.
Electroporation Conditions: The voltage and pulse duration must be carefully optimized for the specific strain of E. coli and the cuvette size. Over-voltage can kill the cells, while under-voltage can result in poor transformation.
Packaging and Amplification of the Phage Library
Once the phage or phagemid vector is inside E. coli, the goal is to produce fully assembled phages that display the inserted proteins:
Helper Phage Co-Infection
In the case of phagemid vectors, a helper phage is required:
The helper phage provides the necessary structural proteins (like pVIII, pIII) but has a defective replication origin to prevent it from outcompeting the phagemid DNA.
M13KO7 is a commonly used helper phage, carrying a defective origin that favors packaging of phagemid DNA into viral particles.
Hyperphages, lacking the gene for pIII, are another choice that forces the use of phagemid-encoded pIII, increasing the accuracy of protein display.
Phage Amplification
Once the E. coli cells are transformed with the phagemid and helper phage, they are grown under specific conditions:
Antibiotic Selection: The phagemid often contains an antibiotic resistance gene (like ampicillin), allowing for the selection of successfully transformed cells.
Incubation at Optimal Temperature: Growth typically occurs at 30°C-37°C, depending on the phage and the insert's stability.
Phage Assembly: Phages are continuously extruded from the E. coli cell membrane, not causing lysis, allowing for high-yield production of phage particles.
Quality Control and Validation of Library Diversity
A constructed library must be evaluated for diversity and quality to ensure it represents a broad set of variants:
Titering the Library
Determining the number of viable phage particles in the library is crucial:
Phage titers are expressed as colony-forming units (CFU) or plaque-forming units (PFU).
High titers indicate a robust library, but it's essential to assess whether the diversity matches the expected variety.
Sequencing Analysis
Random Sampling: A subset of the phage library is sequenced to verify the diversity and the presence of expected inserts. This can be done using traditional Sanger sequencing for a small sample or next-generation sequencing (NGS) for a larger analysis.
Error Rate: DNA sequencing allows for estimating the mutation rate introduced during library construction and confirming that the inserted sequences match the design.
Functional Validation
Biopanning: Test rounds of selection are conducted to ensure that the library contains functional variants capable of binding to target molecules.
Binding Assays: ELISA or other binding assays are performed to quantify the library’s specificity and affinity to the target.
Storage and Maintenance of the Library
Proper storage conditions are necessary to maintain the integrity and viability of the constructed library:
Glycerol Stocks: Bacterial stocks containing the phage library are stored in glycerol at -80°C. Glycerol prevents ice crystal formation, maintaining cell viability.
Phage Stocks: Pure phage preparations can be stored in TBS (Tris-buffered saline) at 4°C for short-term storage or in 50% glycerol at -20°C for long-term storage.
Repeat Amplification: Libraries may require periodic amplification to maintain titers, which should be carefully monitored to avoid genetic drift or loss of diversity.
Constructing a phage display library involves a series of highly technical and precise steps, each of which must be optimized to achieve a broad and functional library. By understanding the details of library diversity, vector preparation, transformation, and amplification, researchers can harness the full power of phage display to explore vast sequence spaces, identify specific binding proteins, and develop new diagnostics and therapeutics.
The Process of Phage Display Selection
Phage display selection, often termed biopanning, is the critical procedure for identifying phages that display high-affinity binding proteins from a vast library of variants. This iterative process enables the isolation of phages with the most specific and high-affinity interactions to a target molecule. Below, I’ll provide a deep technical exploration of the biopanning process, highlighting each phase from preparation to refinement, with a focus on achieving highly specific and effective selections.
Overview of the Biopanning Process
Biopanning involves several rounds of selection to gradually enrich a phage population for the highest-affinity binders. Each round includes binding, washing, elution, amplification, and validation. Here’s a breakdown of the core steps involved:
Preparation of Target for Selection
The first step in phage display selection is preparing the target antigen that will be used to capture specific phage particles. This requires careful consideration of the target's presentation and the surface on which it will be immobilized:
Target Immobilization
Solid Surfaces: The target antigen (e.g., protein, peptide, cell) is typically immobilized on a solid surface to facilitate interaction with the phage:
Microtiter Plates (ELISA Plates): A 96-well plate with high-binding capacity is commonly used. The wells are coated with the target protein.
Magnetic Beads: Paramagnetic beads coated with target antigens allow for easy separation using a magnetic field. This format is highly efficient and versatile, suitable for a wide range of targets.
Plastic Surfaces or Nitrocellulose Membranes: These can also be used, but they may suffer from nonspecific binding issues.
Target Coupling
The target antigen is often chemically or physically attached to the solid surface:
Direct Adsorption: The target protein is passively adsorbed onto the surface. This is the simplest method but may lead to random orientations of the target.
Biotin-Avidin System: Targets are biotinylated and captured on avidin- or streptavidin-coated surfaces, ensuring strong and specific attachment.
Covalent Crosslinking: Crosslinkers like glutaraldehyde are used to create covalent bonds between the target and the surface, stabilizing the antigen and ensuring it remains attached during washing.
Blocking to Prevent Nonspecific Binding
Blocking Agents: Surfaces are blocked with proteins or polymers that occupy nonspecific binding sites, preventing phages from adhering non-selectively. Common blockers include:
Bovine Serum Albumin (BSA).
Skim Milk.
Casein.
Polyethylene Glycol (PEG).
Incubation of Phage Library with the Target
The phage library, containing millions or billions of unique phage variants, is introduced to the immobilized target. This step is critical as it initiates the selective binding:
Incubation Conditions
Temperature: The incubation temperature must be optimized for protein stability. Most selections occur at room temperature (20-25°C), though physiological temperatures (37°C) can be used to simulate in vivo conditions.
Incubation Time: Varies based on the binding kinetics of the target-protein interaction. Short incubations favor fast-binding proteins, while longer incubations allow weak or slow-binding phages to interact.
pH and Salt Concentration: The pH and ionic strength of the buffer can significantly affect binding. Phosphate-buffered saline (PBS) is common, but modifications (e.g., using Tris buffer) may enhance specific interactions or mimic physiological conditions.
Washing: Removing Nonspecific Binders
Once incubation is complete, the next step is to wash away phages that do not specifically bind to the target:
Stringency Control
Buffer Composition: Washing buffers vary in stringency. Early rounds may use mild conditions (e.g., PBS with 0.1% Tween-20), while later rounds increase stringency (e.g., PBS with 0.5% Tween-20 or high-salt buffers) to eliminate weak binders.
Number of Washes: The number of washes increases with each round. Starting with 3-5 washes in the first round and increasing to 10-15 washes in subsequent rounds enhances specificity.
Agitation: Vigorous shaking or vortexing during washes can help remove nonspecific phages, while gentle washing may retain low-affinity binders.
Binding Kinetics
On-Rate and Off-Rate: Binding strength is a combination of how quickly a phage binds to a target (on-rate) and how long it remains bound (off-rate). Later rounds of selection aim to isolate phages with slow off-rates (high affinity), using longer or more aggressive wash steps.
Elution: Releasing Specifically Bound Phages
After washing, the phages that remain bound are considered high-affinity candidates. Elution is the process of detaching these phages for further amplification and analysis:
Elution Methods
Low pH Elution: Acidic buffers (e.g., glycine-HCl at pH 2.2) disrupt protein-protein interactions, releasing the bound phages. Immediately neutralizing the solution is necessary to preserve phage viability.
Competitive Elution: A high concentration of free target (e.g., non-immobilized protein) is added, outcompeting the immobilized target and displacing bound phages. This method favors phages with biologically relevant binding.
Enzymatic Elution: Proteases like trypsin can cleave a linker between the target and the surface, releasing bound phages without denaturing them.
Gentle Elution: High-salt or high-concentration detergent solutions (e.g., 1M NaCl or 0.1% SDS) can weaken ionic or hydrophobic interactions without damaging the phage.
Controlled Elution
The type of elution impacts the quality of the final binders:
Harsh Elution may favor high-affinity binders that can withstand extreme conditions.
Gentle Elution maintains the integrity of weaker interactions, preserving potential medium-affinity binders.
Amplification: Increasing Phage Yield
Once eluted, the phages need to be amplified to provide sufficient quantities for the next round of selection:
Amplification in E. coli
Infecting Host Bacteria: The eluted phages are used to infect E. coli host cells, typically strains like TG1 or XL1-Blue. These bacteria harbor the F-pilus, which is essential for M13 infection.
Antibiotic Selection: The phagemid or phage often contains an antibiotic resistance gene (e.g., ampicillin), allowing selection of transformed bacteria. This step ensures only cells containing the target phage will grow.
Amplification Protocol:
Cultures are incubated in selective media until they reach the mid-log phase (around 0.4 OD600).
Cultures are infected with a helper phage if using a phagemid system, initiating the packaging of single-stranded DNA into phage particles.
Phage particles are continuously extruded from the host cell, harvested, and purified for the next round.
Iterative Rounds of Selection: Increasing Specificity
Biopanning is an iterative process, with each round refining the specificity and affinity of the selected phage population:
Increasing Selection Stringency
Reducing Target Concentration: In successive rounds, the concentration of the immobilized target is decreased, requiring phages to have higher affinity for effective binding.
Tougher Washing Conditions: The wash steps become more stringent with each round, using stronger detergents or higher salt concentrations to remove all but the tightest binders.
Shorter Incubation Times: Shortening the incubation period in later rounds favors fast-on-rate phages, isolating those that quickly bind the target.
Monitoring Enrichment
Phage Titering: Phage titers are monitored at each round to assess the amplification efficiency.
Enrichment Factor: The ratio of recovered phages between rounds indicates enrichment. A significant increase in recovered phages suggests that selection is honing in on high-affinity candidates.
Validation and Characterization of Selected Phages
After completing multiple rounds of biopanning, the selected phage population undergoes validation to identify the best candidates:
DNA Sequencing
Individual clones are sequenced to determine the genetic code of the displayed peptide or protein.
Sequencing can reveal if convergent evolution has occurred, where multiple independent clones converge to similar sequences, suggesting a robust binding interaction.
Functional Assays
ELISA (Enzyme-Linked Immunosorbent Assay): Used to verify the binding of selected phages to the target. ELISA can provide quantitative data on binding strength.
Surface Plasmon Resonance (SPR): Measures real-time binding kinetics, providing detailed data on on-rates and off-rates, crucial for assessing affinity.
Flow Cytometry: For cell-surface antigens, selected phages can be labeled with a fluorescent marker and analyzed for binding specificity to target cells.
Production of Soluble Proteins for Final Analysis
If the final goal is to use selected peptides or proteins outside of a phage context, conversion to soluble form may be necessary:
Expression Vectors: Genes encoding the best candidates are sub-cloned into expression vectors that produce soluble proteins.
Purification: Tags like His-tags or FLAG-tags facilitate purification of soluble proteins for downstream applications.
Affinity Testing: The purified proteins are tested again to confirm that the observed binding properties are retained in the soluble form.
The process of phage display selection is a meticulously controlled procedure that involves multiple rounds of enrichment, amplification, and validation. Each round refines the library, focusing in on high-affinity binders through iterative increases in selection stringency. This method provides a powerful tool to explore large protein spaces, allowing researchers to identify specific interactions for diagnostic, therapeutic, and research purposes.
Analysis and Characterization
Once potential binders are isolated through the phage display selection process, thorough analysis and characterization are critical to ensure the reliability, specificity, and affinity of these molecules. This stage involves a series of detailed biochemical and biophysical assays to validate the selected clones, understand their binding properties, and optimize them for downstream applications. Below, I’ll provide a technical breakdown of each analytical method and characterization technique.
DNA Sequencing of Selected Phage Clones
The first step in the analysis is to determine the sequence of the DNA that encodes the displayed protein:
Sanger Sequencing
Purpose: Used for small-scale verification of individual clones. After several rounds of biopanning, a subset of selected phage clones is sequenced.
Process:
The single-stranded DNA (ssDNA) from the phage or phagemid is isolated.
PCR is performed using primers flanking the DNA insert region (usually the modified pIII or pVIII gene).
Sequencing is conducted using the Sanger method, which provides a clear sequence of the inserted region.
Benefits: High accuracy, cost-effective for analyzing a few samples.
Limitations: Limited throughput—inefficient for analyzing a large number of clones.
Next-Generation Sequencing (NGS)
Purpose: Used for high-throughput sequencing of hundreds to thousands of phage clones, providing a comprehensive overview of the selected library.
Process:
DNA is extracted from a pool of phages after selection.
Adaptors are ligated to the DNA fragments, allowing for sequencing via platforms like Illumina or Ion Torrent.
Data analysis includes alignment to reference sequences and identification of enriched clones.
Benefits: High throughput, provides a deep understanding of diversity and convergence in the selected population.
Limitations: More expensive and requires bioinformatics tools for data processing.
Analysis of Sequencing Results
Clonal Convergence: A successful selection often leads to convergence—where multiple phage clones display similar or identical sequences, suggesting a true high-affinity binder.
Mutational Hotspots: Sequencing analysis can reveal areas of variability, indicating positions critical for target binding.
Diversity Metrics: Shannon entropy or other statistical measures are used to assess the diversity of the library and the extent of enrichment after each biopanning round.
Binding Assays to Confirm Affinity and Specificity
After identifying the DNA sequences, the next step is to verify that the phage-displayed proteins indeed bind to the target antigen with the desired specificity and affinity:
ELISA (Enzyme-Linked Immunosorbent Assay)
Purpose: A colorimetric assay that quantitatively measures the binding interaction between the phage-displayed protein and the target antigen.
Process:
The target antigen is immobilized on a 96-well plate.
Phages displaying the selected proteins are added to the wells.
After incubation and washing, a secondary antibody conjugated to an enzyme (e.g., HRP—Horseradish Peroxidase) that recognizes the phage is added.
A substrate is introduced, producing a color change proportional to the binding interaction.
Data Analysis: Absorbance measurements are used to determine binding strength.
Advantages: Simple, high-throughput, quantitative.
Limitations: Limited ability to determine binding kinetics (only provides endpoint measurements).
Surface Plasmon Resonance (SPR)
Purpose: A biophysical method to study real-time binding interactions, measuring association and dissociation rates to determine binding kinetics.
Process:
The target antigen is immobilized on a sensor chip.
Phage particles or purified proteins are flowed across the chip surface.
A light beam detects changes in the refractive index at the chip’s surface, indicating binding events.
Association (on-rate) and dissociation (off-rate) data are captured in real-time, providing a kinetic profile.
Data Analysis:
On-rate (“k”on): How quickly the protein binds to the target.
Off-rate (“k”off): How quickly the protein dissociates from the target.
Equilibrium Dissociation Constant (K"D"), A ratio of "k"off/"k"on indicating binding affinity. Lower "K"D values indicate higher binding affinity.
Advantages: Provides kinetic details, real-time analysis.
Limitations: Requires expensive instrumentation and precise control of experimental conditions.
Detailed Characterization of Binding Properties
Further characterization is needed to determine the exact specificity, cross-reactivity, and affinity of the selected binders:
Epitope Mapping
Purpose: Identifies the specific regions (epitopes) of the target protein to which the phage-displayed proteins bind.
Techniques:
Peptide Scanning: A series of overlapping synthetic peptides covering the entire target protein sequence are immobilized. Phages are tested for binding to specific peptides.
Alanine Scanning: Critical residues are identified by systematically replacing each amino acid with alanine and assessing the impact on binding.
Advantages: Provides precise binding sites, helps in understanding the mechanism of interaction.
Limitations: Time-consuming if many residues need to be scanned.
Competitive Binding Assays
Purpose: Measures whether the selected phages compete for the same binding site as a known antibody or ligand.
Process:
A known competitor (e.g., an antibody) is pre-incubated with the target.
The phage-displayed proteins are then added, and binding is assessed using ELISA or SPR.
Data Analysis: If binding decreases in the presence of a competitor, the phage displays a protein that binds to the same or overlapping site.
Advantages: Helps in determining binding specificity and potential cross-reactivity.
Limitations: Requires known competitors with established binding sites.
Affinity Maturation: Improving Binding Properties
If the selected proteins have moderate affinity, they can undergo an affinity maturation process to enhance their binding characteristics:
Mutagenesis Approaches
Error-Prone PCR: Introduces random mutations into the DNA encoding the displayed protein, generating variants with potential improvements in affinity.
Site-Directed Mutagenesis: Specific residues identified as critical for binding are systematically mutated to optimize interactions with the target.
Chain Shuffling: For antibodies, swapping variable heavy (VH) and variable light (VL) regions from different clones can generate combinations with improved binding properties.
Screening of Mutated Libraries
The mutated or shuffled variants are screened using biopanning to select clones with improved binding characteristics.
SPR or ELISA is used to validate and compare the affinities of original versus mutated clones.
Thermodynamic Characterization
For a complete understanding of binding properties, thermodynamic parameters can be analyzed:
Isothermal Titration Calorimetry (ITC)
Purpose: Directly measures the heat change during the binding interaction to provide detailed thermodynamic data.
Process:
The target antigen is titrated with the phage-displayed protein or purified binder.
The heat absorbed or released is measured and analyzed to provide binding enthalpy (ΔH), entropy (ΔS), and the equilibrium dissociation constant (KD_DD).
Advantages: Provides complete thermodynamic profile, including binding stoichiometry.
Limitations: Requires larger amounts of purified protein, sensitive to experimental conditions.
Differential Scanning Calorimetry (DSC)
Purpose: Measures the thermal stability of the protein-target complex.
Process:
The complex is heated, and the heat absorption is monitored.
The melting temperature (Tm_mm) indicates complex stability, with higher Tm_mm values suggesting more stable binding.
Advantages: Provides insights into the structural stability of the interaction.
Limitations: Only provides stability data, not specific affinity measurements.
Structural Analysis: Determining 3D Interactions
Understanding the exact 3D structure of the protein-target complex is the gold standard for confirming binding:
X-Ray Crystallography
Purpose: Provides a high-resolution structure of the protein-ligand complex.
Process:
Crystals of the protein-target complex are grown.
X-rays are directed at the crystal, and the diffraction pattern is analyzed to determine the electron density and create a 3D model.
Advantages: Very high resolution (atomic level), allows detailed study of binding interactions.
Limitations: Requires successful crystal formation, which can be challenging.
Cryo-Electron Microscopy (Cryo-EM)
Purpose: Visualizes protein complexes at near-atomic resolution without the need for crystallization.
Process:
The complex is flash-frozen in a thin layer of ice and observed with an electron microscope.
Multiple 2D images are reconstructed into a 3D model using computational methods.
Advantages: Suitable for large and flexible complexes, requires less sample preparation than crystallography.
Limitations: Lower resolution than crystallography, but rapidly improving with advances in technology.
Production and Validation of Soluble Forms
If the goal is to use the selected protein in non-phage contexts (e.g., therapeutic development), the protein is expressed in a soluble form:
Subcloning into Expression Vectors
The gene encoding the selected binder is transferred to an expression vector optimized for soluble protein production in systems like E. coli, yeast, or mammalian cells.
Tags (like His-tag or GST-tag) are added for easy purification.
Protein Purification and Refolding
Proteins are purified using affinity chromatography, ion exchange, or size-exclusion chromatography.
If the protein is expressed in inclusion bodies (aggregates), refolding protocols are employed to restore native conformation.
Functional Validation
Purified proteins are validated for function using the same assays applied to the phage-displayed versions (e.g., ELISA, SPR, ITC).
The analysis and characterization phase is as critical as the selection process itself, providing confirmation of the specificity, affinity, and stability of the selected binders. This stage involves a variety of molecular, biophysical, and structural techniques to ensure that the phage-selected proteins perform as intended, paving the way for their use in diagnostic, therapeutic, or research applications.
Types of Phage Display Systems
Phage display systems have evolved significantly since the original description, leading to various specialized formats tailored to specific research needs. Different systems utilize distinct coat proteins for display, employ various vector types (phage or phagemid), and target different applications, from simple peptide displays to complex protein interactions. Below, I will break down the technical aspects of each major type of phage display system, focusing on the mechanisms, strengths, and limitations of each.
Filamentous Phage Display Systems
Filamentous phage display is the most common type of phage display, utilizing filamentous bacteriophages like M13, fd, or f1. These phages are non-lytic, meaning they extrude from the host bacteria without killing it, allowing for continuous phage production.
Coat Proteins Utilized in Filamentous Phages
The primary coat proteins used in M13-based phage display systems are pIII and pVIII, though pVII and pIX are occasionally employed.
pIII Display:
Location: Found at one end of the phage, with around 3-5 copies per phage particle.
Function: Essential for phage infection of E. coli. It attaches to the F-pilus on the bacterial surface to initiate infection.
Advantages:
Ideal for displaying larger proteins or complex structures since only a few copies are presented, reducing steric hindrance.
Lower avidity effect due to fewer copies, leading to more accurate affinity measurements.
Limitations:
Limited valency (fewer displayed copies), which may reduce sensitivity in low-affinity interactions.
pVIII Display:
Location: The most abundant coat protein, with approximately 2,700 copies per phage, forming the bulk of the filamentous capsid.
Function: Provides the structural backbone for the phage particle.
Advantages:
High valency allows for displaying small peptides in large numbers, enhancing sensitivity in initial screenings.
Effective for epitope mapping or identifying binding motifs.
Limitations:
Restricted to small peptides due to structural constraints. Inserting larger proteins disrupts phage stability.
Strong avidity effect can mask low-affinity interactions, leading to false positives in certain assays.
pVII and pIX Display:
Location: Located at the opposite end of the phage from pIII, with five copies of each protein.
Advantages:
Capable of forming multimeric structures, suitable for displaying complex protein domains or interacting pairs.
Minimal steric hindrance, as pVII and pIX are located at the distal end of the filament.
Limitations: Less commonly used, so fewer standardized protocols are available.
Phagemid-Based Systems
Phagemids are hybrid vectors combining elements of plasmids and phage genomes. They are commonly used in filamentous phage systems:
Phagemid Properties:
Contain a bacterial origin of replication for stable maintenance and a phage origin (f1 Ori) for packaging into phage particles.
Display genes (e.g., pIII or pVIII) can be easily manipulated due to the modular nature of the vector.
Helper Phage Requirement:
A helper phage provides the necessary coat proteins and replication machinery. It preferentially packages the phagemid DNA due to a defective replication origin.
Commonly used helper phages include M13KO7 and Hyperphage.
T7 Phage Display System
The T7 phage display system uses bacteriophage T7, a double-stranded DNA (dsDNA) lytic phage. Unlike filamentous phages, T7 lyses the host cell upon replication, releasing large numbers of phage particles in a burst.
Mechanism of T7 Display
Foreign Protein Insertion: The foreign DNA sequence is inserted directly into the T7 genome, often at the N-terminus or C-terminus of the gene encoding the 10B capsid protein.
Display Protein: The 10B protein is one of the capsid proteins of T7, and the insertion allows the foreign protein to be presented on the phage surface.
High Stability: T7 capsids are highly stable, capable of displaying larger and more structurally complex proteins without affecting phage viability.
Advantages of T7 Phage Display
Higher Structural Stability: The T7 capsid is resistant to harsh conditions like extremes of pH or temperature, allowing for more stringent selection conditions.
High Expression Efficiency: T7 RNA polymerase is robust, leading to high levels of protein expression.
Rapid Screening: Due to the lytic nature of T7, phage production is fast, enabling rapid library screening.
Limitations of T7 Phage Display
Lytic Cycle: The lytic nature means that phage amplification leads to cell death, which limits continuous culture and makes it challenging to scale up.
Limited Valency: The number of displayed proteins per phage is lower compared to M13-based systems, potentially reducing sensitivity in early rounds of selection.
Lambda (λ) Phage Display System
The Lambda (λ) phage is another dsDNA phage system, historically significant but less commonly used than M13 or T7 systems.
Mechanism of λ Phage Display
Fusion Proteins: Foreign proteins are inserted as fusions to gpD, a minor coat protein, or gpV, a major tail protein.
gpD: Provides higher stability for displayed proteins, suitable for larger protein domains.
gpV: A minor component but can support multivalent display.
Lysogenic and Lytic Phases: Lambda phage can exist in both a lytic cycle (where it lyses the host) or a lysogenic state (integrating into the host genome), providing flexibility for different experimental needs.
Advantages of λ Phage Display
Stable Genome: The large dsDNA genome can accommodate more complex inserts compared to single-stranded phage systems.
Versatile Lifecycle: The ability to switch between lysogenic and lytic cycles provides control over phage propagation.
High Expression Levels: The lambda phage's strong promoters ensure robust expression of displayed proteins.
Limitations of λ Phage Display
Less Common: There are fewer commercially available vectors and kits for lambda phage display compared to M13.
Complex Protocols: The lifecycle control requires more specialized knowledge and handling.
Yeast Display Systems (Alternative to Phage Display)
While not a phage system, yeast display is worth mentioning as it shares similarities with phage display in its purpose of displaying peptides or proteins on the surface of a biological entity. Yeast display uses Saccharomyces cerevisiae to display proteins on the yeast cell surface.
Mechanism of Yeast Display
Aga2p Fusion: Proteins of interest are fused to Aga2p, a protein that anchors to the yeast cell wall through interaction with Aga1p.
FACS Screening: Yeast display enables fluorescence-activated cell sorting (FACS), a highly quantitative method for selecting binders based on fluorescence labeling.
Advantages of Yeast Display
Eukaryotic Folding: The eukaryotic system supports proper folding, post-translational modifications, and disulfide bond formation, which is essential for complex proteins like antibodies.
Quantitative Screening: FACS provides detailed data on binding strength and kinetics, enabling fine discrimination between candidates.
Limitations of Yeast Display
Slower Growth: Yeast cultures grow slower than bacterial systems, making library screening less rapid.
Limited Diversity: Transformation efficiency is lower compared to phage systems, limiting library diversity.
Ribosome Display and mRNA Display (Alternative In Vitro Systems)
These are in vitro display methods that do not rely on living cells or phages, allowing the display of proteins or peptides in a cell-free environment.
Ribosome Display
Mechanism: The mRNA encoding the protein of interest is translated in a cell-free system, but the ribosome is not released. This results in a complex of ribosome, mRNA, and nascent peptide.
Advantages:
No Transformation Limitations: Avoids the transformation step, allowing extremely large libraries (10¹³ or more variants).
Rapid Cycles: Faster screening as there is no need for cell culture or phage propagation.
Limitations:
Lack of Stability: Ribosomes are sensitive to degradation, and handling requires precise conditions.
No Post-Translational Modifications: As an in vitro system, lacks the capacity for eukaryotic-like modifications.
mRNA Display
Mechanism: The protein of interest is linked to its encoding mRNA via a puromycin linker. When translated in vitro, the nascent protein remains attached to the mRNA, enabling genotype-phenotype linkage.
Advantages:
Ultra-Large Libraries: Capable of producing libraries with extremely high diversity.
Direct Sequence Analysis: Selected mRNA can be directly reverse-transcribed and sequenced.
Limitations:
Technical Complexity: Requires specialized protocols and equipment.
Handling Challenges: RNA stability is a concern, requiring careful experimental design.
Comparison of Phage Display Systems
Different types of phage display systems, from the traditional filamentous M13 to the robust T7 system, offer a spectrum of options tailored to the specific needs of a research project. Each system has strengths and weaknesses based on the target protein size, desired stability, selection stringency, and scalability. By understanding these distinctions, researchers can choose the optimal display platform for their specific goals, whether it's antibody discovery, epitope mapping, or novel protein engineering.
Applications of Phage Display
Phage display is a versatile technology with a wide range of applications across multiple fields, including drug discovery, diagnostic development, vaccine design, and molecular biology research. By leveraging the ability of bacteriophages to present diverse peptide and protein libraries on their surface, researchers can identify specific interactions and optimize molecules with high precision. Below, I’ll delve into the technical specifics of key applications, highlighting how phage display is used and optimized for each purpose.
Drug Discovery: Screening for High-Affinity Ligands
Phage display is widely used in drug discovery for identifying peptides, proteins, and small molecules that bind with high specificity to therapeutic targets:
Antibody Discovery and Optimization
Objective: Identify and engineer monoclonal antibodies that target specific proteins or pathogens with high affinity and specificity.
Method:
Antibody Libraries: Libraries are constructed using the variable regions of antibodies, especially the heavy (VH) and light (VL) chains, which determine specificity.
Single-Chain Variable Fragments (scFv) and Fab Fragments: These antibody fragments are displayed on phage surfaces, allowing rapid screening for specific antigen binding.
Affinity Maturation: Selected antibodies undergo iterative rounds of mutagenesis and selection to increase binding affinity. Techniques like error-prone PCR and chain shuffling are used to introduce variations.
Validation:
ELISA and Surface Plasmon Resonance (SPR): Determine binding specificity and kinetics of selected antibodies.
Therapeutic Candidates: High-affinity candidates are converted to full-length antibodies and validated for activity in in vitro and in vivo models.
Peptide Mimics and Inhibitors
Objective: Identify small peptides that mimic the structure of natural ligands or inhibit specific protein-protein interactions.
Method:
Random Peptide Libraries: Libraries of short peptides (e.g., 7-15 residues) are displayed on phage surfaces, providing a vast array of potential binding sequences.
Competitive Screening: Peptides are selected based on their ability to bind to a target in the presence of a known ligand, ensuring that selected peptides mimic or block the natural interaction site.
D-amino Acid Screening: Modified libraries containing D-amino acids are used to find protease-resistant peptide inhibitors.
Applications:
Receptor Antagonists: Identify peptides that block receptor-ligand interactions, reducing disease-related signaling pathways.
Protein Interaction Modulators: Screen for peptides that disrupt key protein-protein interactions in diseases like cancer or inflammation.
Diagnostics: Development of High-Specificity Detection Reagents
Phage display is essential for developing diagnostic tools that detect specific pathogens, biomarkers, or disease states with high sensitivity:
Diagnostic Antibodies and Detection Kits
Objective: Develop antibodies that can specifically bind to disease biomarkers for use in diagnostic tests, like ELISA or lateral flow assays.
Method:
Immune Repertoire Libraries: Antibody libraries derived from immunized animals or human donors provide natural diversity for disease-specific targets.
Non-Immune Synthetic Libraries: Synthetic libraries are designed to target conserved epitopes of viral or bacterial proteins.
Phage-Based ELISA: Direct detection of target antigens using phage-displayed antibodies instead of traditional antibodies, simplifying and reducing costs.
Applications:
Infectious Diseases: Rapid identification of viral or bacterial pathogens (e.g., COVID-19, HIV, influenza) using high-affinity antibodies.
Cancer Biomarkers: Discovery of tumor-specific markers like PSA for prostate cancer or HER2 for breast cancer.
Phage Display for Epitope Mapping
Objective: Identify specific regions (epitopes) on an antigen that are recognized by antibodies, aiding in the design of targeted diagnostics.
Method:
Overlapping Peptide Libraries: Short peptides spanning the entire length of the target antigen are displayed on phage surfaces. Binding studies reveal which regions are recognized by specific antibodies.
Alanine Scanning: Systematic mutation of residues to alanine in the peptide library to identify critical residues for binding.
Applications:
Autoimmune Diseases: Identify autoimmune epitopes targeted by the patient's immune system.
Allergen Identification: Map regions of allergens that trigger immune responses in patients, aiding in the development of diagnostic tests.
Vaccine Development: Design and Optimization
Phage display plays a critical role in vaccine development, from identifying immunogenic epitopes to optimizing antigen presentation for effective immune responses:
Epitope Discovery for Vaccine Antigens
Objective: Identify peptide sequences that can elicit a robust immune response and serve as effective vaccine components.
Method:
Linear and Conformational Epitope Mapping: Screening phage-displayed libraries with sera from immunized animals or patients to identify epitopes that induce neutralizing antibodies.
Mimotope Discovery: Identification of peptides (mimotopes) that mimic the structure of native antigens, serving as potential vaccine candidates.
Conformational Libraries: Use of libraries that maintain the 3D conformation of antigens, crucial for recognizing epitopes that depend on tertiary structure.
Applications:
Viral Vaccines: Identification of conserved viral epitopes that provide cross-protection against multiple strains.
Cancer Vaccines: Discovery of tumor-associated antigens that stimulate a targeted immune response against cancer cells.
Immunogenicity Enhancement Using Phage Display
Objective: Optimize the presentation of antigens to enhance their immunogenicity.
Method:
Multivalent Display: High valency of phage display (e.g., using pVIII) enhances immune recognition, mimicking natural pathogen structures.
Carrier Phage Particles: Use phages themselves as carriers for vaccine delivery. Phages are inherently immunogenic, providing an adjuvant effect.
Fusion Constructs: Display antigens fused to immunostimulatory molecules (e.g., cytokines) to enhance immune activation.
Applications:
Universal Influenza Vaccines: Identification of conserved epitopes to provide broad protection against multiple flu strains.
Immunotherapy: Creation of personalized cancer vaccines based on tumor-specific antigen discovery.
Protein-Protein Interaction Studies: Molecular Biology Research
Phage display is a powerful tool for studying protein-protein interactions, revealing how proteins interact in cellular processes, and identifying potential inhibitors:
Mapping Protein Interaction Domains
Objective: Identify the domains or motifs within proteins that mediate specific interactions.
Method:
Domain Libraries: Libraries displaying truncated or mutated versions of a protein, allowing researchers to pinpoint the interaction regions.
Peptide Libraries: Display short peptides that mimic the binding regions of full-length proteins.
Yeast Two-Hybrid Alternatives: Phage display offers an alternative to yeast two-hybrid screening by allowing interactions to be screened in vitro without requiring cell-based assays.
Applications:
Signal Transduction Pathways: Map the interactions of signaling proteins like kinases or receptors.
Transcription Factor Binding: Identify peptides that inhibit transcription factor-DNA interactions, useful in gene regulation studies.
Identification of Binding Motifs and Specificity Determinants
Objective: Understand which amino acid residues are critical for specific protein-protein interactions.
Method:
Randomized Mutagenesis Libraries: Libraries are constructed with randomized positions within a peptide sequence to determine which residues are important for binding.
Negative Selection: Use phage display to identify peptides that do NOT bind to a particular target, helping to identify specificity determinants.
Applications:
Inhibitor Design: Create peptides that specifically block undesired protein interactions, such as those in cancer pathways.
Protein Engineering: Use binding motif data to engineer proteins with enhanced or modified functionalities.
Enzyme Engineering: Optimization of Catalytic Function
Phage display facilitates the discovery and optimization of enzymes with improved catalytic properties, expanding their use in industrial and medical applications:
Directed Evolution of Enzymes
Objective: Identify mutations that enhance enzyme activity, specificity, stability, or other desirable properties.
Method:
Error-Prone PCR: Introduces random mutations into the gene encoding the enzyme, generating a diverse library of enzyme variants.
DNA Shuffling: Fragments of multiple enzyme variants are shuffled to create hybrids, combining beneficial mutations.
Substrate Display: Phage libraries displaying enzyme variants are screened for their ability to interact with immobilized substrates, mimicking catalytic conditions.
Applications:
Thermostable Enzymes: Identify mutations that increase enzyme stability at high temperatures, useful for industrial applications.
Enzyme Inhibitors: Discovery of peptide inhibitors that specifically target enzyme active sites, regulating catalytic activity.
Identification of Enzyme Substrates
Objective: Discover novel substrates or cofactors that interact with enzymes.
Method:
Substrate Phage Libraries: Libraries of small molecules or peptides are displayed to identify those that act as substrates or interact with the enzyme’s active site.
Competitive Screening: Enzyme variants are screened in the presence of known substrates to identify those with altered specificity or enhanced binding.
Applications:
Biosensors: Engineering enzymes to recognize specific substrates for diagnostic purposes.
Biocatalysis: Design enzymes for efficient catalysis of specific reactions, such as those needed in green chemistry.
Materials Science: Development of Biomaterials
Phage display is used to identify peptides that bind to non-biological surfaces, aiding in the development of novel biomaterials:
Identification of Surface-Binding Peptides
Objective: Discover peptides that bind specifically to materials like metals, ceramics, or polymers for surface modification or material synthesis.
Method:
Material-Coated Plates: Phage libraries are screened against plates coated with the target material, selecting phages that show specific binding.
Iterative Rounds: Phages are enriched through multiple rounds of biopanning, refining the selection for highly specific binders.
Applications:
Nanotechnology: Identify peptides that bind to nanoparticles, enabling targeted drug delivery or imaging.
Tissue Engineering: Discovery of peptides that enhance cell adhesion to scaffolds, improving biocompatibility for implants.
Phage as a Scaffold for Material Synthesis
Objective: Use phages themselves as scaffolds for the synthesis of nanomaterials, leveraging their ability to display specific binding motifs.
Method:
Metal Nanoparticle Synthesis: Phage-displayed peptides are used to nucleate and grow nanoparticles of metals like gold or silver.
Biotemplating: The filamentous structure of M13 phage is used as a template for assembling complex structures at the nanoscale.
Applications:
Conductive Materials: Create conductive films or nanowires for electronic devices.
Biosensors: Develop biosensors by integrating phage-displayed peptides that bind selectively to biological targets with electronic readout systems.
Phage display is a multifaceted technology with applications spanning drug discovery, diagnostics, vaccine development, protein engineering, and materials science. Each application leverages the core strength of phage display—rapid and robust selection from a vast library of variants to identify high-affinity binders or functional molecules. This adaptability makes phage display a cornerstone technique in modern biotechnology and molecular biology.
Limitations and Challenges
While phage display is a powerful and versatile technology, it is not without its limitations and challenges. These challenges can impact the efficiency, accuracy, and reliability of the selection process, and addressing them requires careful experimental design and optimization. Below, I’ll explore the technical details of the key limitations and challenges associated with phage display, along with potential solutions.
Library Design Limitations
The construction of a diverse and high-quality library is a fundamental aspect of phage display. However, achieving sufficient diversity and avoiding biases can be challenging.
Limited Library Diversity
Problem: The theoretical diversity of a phage display library can reach billions of variants, but practical limitations often reduce this number:
Transformation Efficiency: The efficiency of introducing DNA into E. coli cells (via electroporation or chemical transformation) limits the maximum number of variants that can be represented in a library.
Clonal Amplification: During amplification, some clones may outcompete others, leading to an overrepresentation of certain sequences and reduced effective diversity.
Technical Details:
Transformation efficiency for E. coli typically peaks at 10⁸ to 10¹⁰ colony-forming units (CFU) per microgram of DNA. For libraries larger than 10⁹ variants, multiple transformations may be needed to achieve sufficient coverage.
Competent cell preparation, electroporation conditions, and DNA purity all significantly influence transformation efficiency.
Solutions:
High-Efficiency Competent Cells: Use commercially available cells optimized for high transformation efficiency.
DNA Preparation: Optimize DNA purity and concentration, ensuring minimal contaminants that could interfere with transformation.
Pooling Transformations: Use multiple parallel transformations and pool them to increase the overall library diversity.
Codon Bias and Expression Issues
Problem: The expression of foreign proteins in E. coli can be hindered by differences in codon usage between the gene source and the bacterial host:
Codon Bias: Some codons are rarely used in E. coli, leading to inefficient translation and reduced protein expression.
Secondary Structure Formation: Certain DNA sequences can form secondary structures (like hairpins) that impede transcription or translation.
Technical Details:
Codon optimization is critical when synthesizing genes for phage display. The use of synonymous codons preferred by E. coli can enhance expression efficiency.
Solutions:
Codon Optimization: Use online tools or commercial services to optimize codon usage for E. coli.
Ribosome Binding Site Adjustment: Ensure that the ribosome binding site (RBS) in the expression construct is compatible with E. coli for efficient translation initiation.
Affinity Selection and Biopanning Challenges
The biopanning process, used to enrich phage populations for high-affinity binders, is central to phage display but presents several technical challenges.
Nonspecific Binding and Background Noise
Problem: Phages can bind nonspecifically to surfaces, blockers, or target proteins, generating background noise and reducing the accuracy of selection.
Technical Details:
The phage coat proteins (especially pIII and pVIII) can interact non-specifically with the target or solid support, leading to false-positive binders.
Non-specific interactions can be exacerbated by hydrophobic or electrostatic forces between the phage and the surface.
Solutions:
Blocking Agents: Use efficient blocking agents like BSA, skim milk, or PEG to minimize nonspecific binding. The choice of blocking agent should match the chemistry of the target and surface.
Washing Stringency: Increase the number and stringency of washing steps in later rounds of biopanning to reduce background noise.
Negative Selection: Use control surfaces or irrelevant targets for negative selection to remove phages that bind nonspecifically.
Avidity Effects
Problem: Phage particles can display multiple copies of a target-binding peptide or protein, leading to enhanced apparent affinity due to the avidity effect (multiple simultaneous interactions):
This can mask weak or low-affinity interactions, resulting in the selection of binders that appear to have high affinity but do not in practice.
Technical Details:
Avidity is particularly prominent in multivalent systems like pVIII-based displays, where thousands of copies of the same peptide are presented per phage.
Solutions:
Monovalent Display Systems: Use monovalent display formats (e.g., pIII-based systems) where only a few copies of the protein are displayed per phage. This approach minimizes the avidity effect, allowing a more accurate assessment of true affinity.
Biophysical Characterization: Use post-selection biophysical assays (like SPR) to confirm true binding affinities without the influence of avidity.
False Positives and False Negatives
False positives (non-specific binders that appear specific) and false negatives (missing true binders) are persistent challenges in phage display.
False Positives
Problem: False positives can arise due to non-specific interactions during the selection process or the ability of certain phages to bind to the target regardless of displayed peptide specificity.
Technical Details:
Some phage clones may exhibit strong electrostatic or hydrophobic interactions with the target that are independent of the displayed peptide sequence.
Solutions:
Negative Biopanning: Include a negative selection step where phages are pre-incubated with irrelevant targets or surfaces to eliminate non-specific binders.
Counter-Selection: Use structurally similar but non-target proteins in selection rounds to filter out phages that bind to common features rather than specific epitopes.
False Negatives
Problem: High-affinity binders can sometimes be missed if they do not survive stringent selection conditions or if they are outcompeted by lower-affinity, fast-binding phages.
Technical Details:
Certain epitopes may be masked or inaccessible if they require conformational flexibility that the phage display system does not adequately represent.
Solutions:
Multiple Selection Conditions: Use a range of biopanning conditions (e.g., different pH, salt concentrations, or buffer compositions) to capture a broader range of binders.
Low Stringency Initial Rounds: Begin with low-stringency conditions to capture a wider pool of binders before increasing stringency in later rounds.
Phage Display Format-Specific Challenges
Different phage display systems, such as M13, T7, and λ, have inherent challenges based on their biology and structure.
M13 and Filamentous Phages
Problem: Filamentous phages like M13 are highly sensitive to harsh selection conditions, which can limit the types of targets that can be screened:
pIII Degradation: The pIII protein is crucial for infection, and harsh conditions can degrade it, leading to loss of infectivity and reduced recovery of high-affinity clones.
Structural Constraints: Larger or complex proteins can disrupt the structure of the filamentous phage, leading to instability or aggregation.
Technical Details:
The M13 phage's non-lytic life cycle means it continuously extrudes phage particles, allowing for sustained propagation but can be susceptible to structural disturbances.
Solutions:
Gentle Elution Conditions: Use gentle elution buffers (high salt, mild detergents) to minimize damage to pIII during selection.
Alternative Display Proteins: Explore pVII or pIX for displaying larger proteins, as these regions are less structurally constrained.
T7 and Other Lytic Phages
Problem: Lytic phages like T7 have rapid replication cycles that can lead to a high turnover of phage particles, increasing the chance of losing low-abundance clones during amplification.
Technical Details:
T7 phage displays are robust but do not tolerate large inserts well due to the rigid capsid structure.
Solutions:
Pre-Amplification: Use pre-amplification of eluted phages before stringent selection rounds to avoid losing low-abundance, high-affinity candidates.
In Silico Filtering: Use computational tools to simulate and predict the stability of target inserts within the T7 capsid before library construction.
Library Stability and Genetic Drift
Maintaining the integrity of the phage library over multiple rounds of selection is essential to avoid genetic drift or loss of diversity.
Genetic Stability of Displayed Proteins
Problem: Repeated propagation of phages can introduce mutations in the displayed protein region, leading to drift from the original library diversity.
Technical Details:
Phagemid-based systems using helper phages are more prone to genetic instability, as the phagemid can accumulate mutations over successive amplification rounds.
Solutions:
Short Rounds of Selection: Minimize the number of selection and amplification cycles to reduce the chance of accumulating mutations.
High-Fidelity DNA Polymerases: Use high-fidelity DNA polymerases during PCR steps to minimize the introduction of random mutations.
Incomplete Packaging and Contaminant DNA
Problem: Helper phages used in phagemid systems can sometimes be packaged alongside the target phagemid DNA, introducing contaminant phages and reducing library purity.
Technical Details:
This contamination often results in lower-quality selection outputs, as irrelevant phage particles can persist through rounds of biopanning.
Solutions:
Selective Helper Phages: Use helper phages with defective packaging signals (like M13KO7) that favor the packaging of phagemid DNA over the helper's own DNA.
Hyperphage: Employ hyperphage systems, which lack key proteins, forcing packaging of only the target phagemid.
Technical Handling Challenges
There are practical challenges in handling phage display experiments, from maintaining phage viability to scaling up library production.
Phage Stability and Storage
Problem: Phage particles, especially filamentous types, can lose infectivity over time if not stored properly, impacting reproducibility.
Technical Details:
Freeze-thaw cycles can significantly reduce the viability of phages, and improper buffer conditions can lead to aggregation or degradation.
Solutions:
Proper Storage Conditions: Store phages in glycerol stocks at -80°C for long-term preservation or in 4°C for short-term use with appropriate buffers (e.g., TBS or PBS with additives like 10% glycerol).
Avoid Freeze-Thaw Cycles: Aliquot phage stocks to avoid repeated freeze-thaw cycles.
Scaling Up for High-Throughput Screening
Problem: Scaling up phage display for industrial applications or large-scale screenings presents logistical and technical challenges.
Technical Details:
High-throughput screening requires automated liquid handling, robotics for ELISA, and advanced data analysis tools to handle large datasets.
Solutions:
Automation: Integrate liquid-handling robotics for biopanning and ELISA to increase reproducibility and throughput.
Data Management: Use bioinformatics tools to manage and analyze the large datasets generated during high-throughput selections.
Phage display, while immensely powerful, requires careful consideration of its limitations and challenges. From library construction to the biopanning process, each step has inherent technical difficulties that must be addressed to ensure successful selection outcomes. Solutions often involve balancing experimental design, adjusting protocols, and employing specialized reagents to mitigate these challenges. Advanced techniques continue to evolve to overcome these limitations, making phage display an ever-improving technology for molecular biology and biotechnology applications.
Technical Advances and Variations
Phage display technology has undergone numerous innovations since its inception, driven by the need to overcome inherent limitations and expand its applications. These technical advances range from modifications in phage and phagemid systems to the development of completely new display platforms. Below, I’ll dive into the technical details of key advances and variations in phage display, highlighting how they enhance the efficiency, versatility, and accuracy of this powerful tool.
Phage Modifications and Specialized Phage Systems
One of the major areas of advancement in phage display has been the modification of phage vectors and coat proteins to optimize the presentation of foreign peptides and proteins.
Hyperphage Systems
Background: Hyperphages are specially engineered helper phages that lack the gene for the pIII coat protein, forcing the displayed protein to originate exclusively from the phagemid DNA.
Technical Details:
Lack of Native pIII: The hyperphage lacks functional pIII, so only pIII encoded by the phagemid is available for phage assembly. This ensures that the target protein is displayed exclusively on the phage surface.
Effect on Display: This results in higher display efficiency for pIII-based systems, as every phage particle will express the target protein, reducing background contamination.
Advantages:
Higher Display Levels: Allows for more consistent and accurate pIII-based display.
Improved Affinity Selections: Better for selecting high-affinity clones due to reduced noise from background pIII expression.
Limitations:
Restricted to pIII-Based Display: Not suitable for pVIII or other coat protein-based displays.
Split-Protein Systems for Improved Display
Background: In split-protein systems, the displayed protein is divided into two or more fragments that are independently expressed but reassemble on the phage surface.
Technical Details:
Complementation Approach: The protein fragments are expressed in such a way that they complement each other when displayed, allowing large or complex proteins to fold correctly.
Application: Commonly used for displaying large enzymes, antibodies, or multi-domain proteins that may not fold correctly when expressed as a single fragment.
Advantages:
Improved Folding: Enhances the correct folding of large or multi-domain proteins.
Greater Stability: Minimizes misfolding and aggregation issues.
Limitations:
Assembly Dependency: Requires precise control over the expression of fragments to ensure proper assembly on the phage.
Phagemid Enhancements
Double Display Phagemids: Specialized phagemid vectors have been developed to allow dual displays, such as presenting two different peptides on pIII and pVIII simultaneously, facilitating studies of protein-protein interactions.
Phage-Antibody Fusion Systems: Fusion systems that combine phage display with other screening technologies, such as yeast display or mammalian cell display, allow for multi-platform selections that combine the benefits of different systems.
Affinity Maturation and Library Refinement Techniques
Affinity maturation involves modifying initial binders to increase their binding affinity to a target. This can be achieved through multiple technical strategies, often employing advanced mutagenesis and library refinement methods.
Error-Prone PCR
Background: Error-prone PCR is a technique used to introduce random mutations into the gene encoding the displayed protein, allowing for the creation of a diverse pool of mutants for affinity maturation.
Technical Details:
Controlled Error Rate: Conditions are adjusted (e.g., using imbalanced dNTP concentrations or adding Mn²⁺ ions) to increase the error rate of DNA polymerase during PCR.
Generation of Mutant Libraries: Creates a library of protein variants with subtle differences, some of which may exhibit higher affinity.
Advantages:
Rapid Generation of Diversity: Quickly produces a library of variants around a lead candidate.
Fine-Tuning Affinity: Effective for optimizing affinity without changing the overall structure.
Limitations:
Random Nature: Mutations are not targeted, potentially leading to non-beneficial or disruptive changes.
DNA Shuffling (Molecular Breeding)
Background: DNA shuffling involves fragmenting the gene of interest and reassembling it randomly to create chimeric proteins that may have beneficial mutations.
Technical Details:
Process: DNA fragments are generated by restriction enzymes or DNase digestion, and then reassembled using a high-fidelity polymerase. The fragments recombine randomly, combining beneficial mutations from multiple parents.
Library Size: Shuffling can generate large libraries that explore diverse sequence combinations.
Advantages:
Combines Beneficial Mutations: Enhances the chances of obtaining high-affinity variants by combining advantageous mutations from different clones.
Mimics Natural Evolution: Provides a method to evolve proteins in vitro that mimics natural recombination.
Limitations:
Complexity: Requires careful control to avoid loss of beneficial traits during reassembly.
In Vitro Evolution and Display Alternatives
Advances in in vitro evolution techniques allow researchers to bypass some of the challenges associated with in vivo systems like phage display. New display technologies have emerged to provide complementary strengths.
Ribosome Display
Background: Ribosome display is a cell-free system where the ribosome-mRNA-peptide complex is stabilized without dissociation, allowing for the direct linkage of phenotype (protein) to genotype (mRNA).
Technical Details:
Translation System: Uses a cell-free translation system where mRNA is translated into protein, and the nascent peptide remains attached to the ribosome due to the absence of a stop codon.
Selection: Ribosome complexes are incubated with the target, and bound complexes are collected. The mRNA from bound complexes is amplified using RT-PCR for subsequent rounds of selection.
Advantages:
No Transformation Step: Avoids limitations of bacterial transformation, allowing for ultra-large libraries (10¹²–10¹³ variants).
Rapid Screening: Faster than traditional phage display due to the absence of bacterial culture and amplification steps.
Limitations:
Stability Issues: Ribosome complexes are sensitive to degradation, requiring careful handling.
No Post-Translational Modifications: Cannot perform eukaryotic modifications like glycosylation.
mRNA Display
Background: mRNA display creates a direct physical link between the mRNA sequence and the encoded protein using a puromycin linker.
Technical Details:
Puromycin Mechanism: A puromycin molecule is attached to the 3' end of the mRNA. During translation, puromycin enters the ribosome and forms a covalent bond with the nascent peptide, linking the mRNA to the protein.
Selection: Proteins are selected based on binding affinity, and their attached mRNA is amplified by RT-PCR.
Advantages:
Ultra-High Library Diversity: Can reach diversity levels of 10¹⁴–10¹⁵ variants.
Direct Genotype-Phenotype Link: Simplifies identification of high-affinity binders.
Limitations:
Handling Challenges: RNA stability requires careful experimental design to prevent degradation.
In Vitro Limitation: No cell-based verification of protein function, potentially missing important biological context.
Post-Selection Refinement and Characterization
Refinement of phage display systems involves improving selected candidates' properties, stability, and functionality through various post-selection methods.
Computational Design and Directed Evolution
Background: Computational tools can simulate protein-protein interactions and predict beneficial mutations for enhancing binding affinity.
Technical Details:
In Silico Modeling: 3D structures of selected proteins are modeled using software like Rosetta, PyMOL, or Chimera to identify hot spots for binding.
Rational Design: Site-directed mutagenesis is employed to modify specific residues predicted to enhance affinity or stability.
Virtual Screening: Computational docking studies predict potential mutations or identify new targets for the displayed library.
Advantages:
Precision: Targets specific regions of interest, reducing the randomness of traditional mutagenesis.
Speed: Accelerates the optimization process by focusing on high-potential candidates.
Limitations:
Computational Complexity: Accurate modeling requires substantial computational resources.
Experimental Validation: Predicted improvements require experimental confirmation, which can be labor-intensive.
Epitope-Focused Library Design
Background: Focused libraries reduce the overall diversity by concentrating on a known binding site or epitope, speeding up the selection process for high-affinity binders.
Technical Details:
Library Bias: Libraries are designed with specific amino acid positions randomized, while other regions remain constant to maintain structure.
Targeted Biopanning: Selections are carried out with a specific region of the target protein immobilized, ensuring that binders specifically target a known epitope.
Advantages:
Efficiency: Increases the likelihood of finding binders to a particular region of interest.
Less Screening Required: Focused libraries reduce the number of selection rounds needed.
Limitations:
Bias Risk: Reducing diversity might overlook unexpected high-affinity regions outside the known epitope.
High-Throughput Screening and Automation
Automation has revolutionized phage display by allowing high-throughput selections and characterizations. This requires sophisticated robotic systems and advanced screening techniques.
High-Throughput ELISA and SPR
Background: Screening thousands of candidates efficiently requires automation, especially for large-scale library screens.
Technical Details:
Automated ELISA: Robotic liquid handlers prepare and process ELISA plates, streamlining the screening of hundreds of phage samples simultaneously.
SPR Arrays: Automated surface plasmon resonance (SPR) systems with array-based chips enable simultaneous kinetic measurements of multiple binders.
Advantages:
Scalability: High-throughput systems enable the screening of massive libraries without compromising accuracy.
Real-Time Kinetics: SPR provides detailed kinetic data, allowing for rapid comparison of binding affinities.
Limitations:
Cost: High-throughput systems require significant investment in equipment.
Data Management: Large datasets need robust bioinformatics tools for analysis.
Deep Sequencing for Library Analysis
Background: Next-generation sequencing (NGS) has become an invaluable tool for analyzing phage display libraries, allowing researchers to track selection progression and library diversity.
Technical Details:
Pre- and Post-Selection Sequencing: Libraries are sequenced before and after each round of biopanning to monitor changes in clone frequency and diversity.
Variant Enrichment: Sequencing identifies which variants become enriched through selection, providing insights into binding specificity.
Advantages:
Detailed Analysis: Reveals the diversity of libraries, convergence of selection, and evolutionary paths of high-affinity binders.
Optimization: Helps in refining library construction by identifying underrepresented but valuable clones.
Limitations:
Data Volume: NGS generates vast amounts of data, requiring advanced analysis tools and bioinformatics expertise.
The technical advances in phage display have significantly expanded its capabilities, enabling more efficient selections, higher affinities, and better characterization of binders. From novel phage systems to computational refinement and high-throughput screening, each advancement addresses specific limitations and opens new possibilities for applications in drug discovery, diagnostics, and beyond.
Conclusion
Phage display has solidified its position as a transformative tool in molecular biology, revolutionizing our capacity to study, manipulate, and exploit protein interactions on a molecular scale. Through its innovative use of bacteriophages to link phenotype with genotype, phage display allows for the direct visualization of protein characteristics encoded by specific DNA sequences, streamlining the identification and optimization of high-affinity ligands, monoclonal antibodies, and novel peptides. This technology has advanced drug discovery, diagnostics, vaccine development, and protein engineering, providing a robust platform that has fundamentally reshaped modern biotechnology and molecular research.
The complexity and versatility of phage display stem from its deep integration with the genetic and structural biology of bacteriophages. Filamentous phages, like M13, and lytic systems, such as T7 and λ, each offer unique advantages that cater to different experimental needs. M13, a non-lytic phage, facilitates continuous library production, which is ideal for iterative rounds of biopanning where high-throughput, continuous selection is paramount. Its flexible display system, leveraging coat proteins like pIII and pVIII, allows for variable valency—a critical feature that influences the balance between avidity and specificity in ligand selection. T7 phage, with its double-stranded DNA genome and high capsid stability, withstands harsh conditions, allowing for stringent biopanning protocols that focus on isolating only the highest-affinity interactions. The λ phage, meanwhile, offers a balance between stability and flexibility, allowing researchers to utilize its dual lysogenic and lytic lifecycle for controlled expression and selection cycles.
At the heart of phage display is the careful construction of phage libraries—a process that involves the precise design and manipulation of DNA sequences to ensure both diversity and expression efficiency. Constructing a diverse library capable of representing billions of variants is a technically demanding task. It requires overcoming transformation efficiency limitations in E. coli, mitigating codon biases, and managing clonal competition that can skew the representation of variants during amplification. Techniques like codon optimization, ribosome binding site enhancement, and synonymous codon usage are essential to ensure that foreign proteins are expressed accurately and folded correctly. The introduction of genetic diversity through methods such as error-prone PCR, DNA shuffling, and site-directed mutagenesis is crucial for exploring a wide sequence space and facilitating affinity maturation—allowing researchers to identify and optimize candidate molecules that exhibit enhanced binding properties.
Biopanning, the iterative selection process at the core of phage display, is a highly sophisticated method designed to refine a vast initial pool of variants down to a select few with the highest affinity for the target. This multi-step procedure begins with the careful preparation of the target antigen and its immobilization on solid supports using methods like biotin-avidin linkage or covalent cross-linking. The library is incubated under optimized conditions, with parameters such as buffer composition, incubation time, temperature, and ionic strength being fine-tuned to favor specific interactions. Successive rounds involve increasingly stringent washing steps to eliminate nonspecific binders and low-affinity variants, enhancing the selectivity of the binding population. A key challenge lies in mitigating avidity effects—where multivalent displays can artificially enhance apparent affinity—by employing monovalent display strategies or post-selection analysis to accurately gauge true binding affinities.
Post-biopanning analysis is an integral phase of phage display, involving detailed validation of selected binders. DNA sequencing, whether through traditional Sanger methods or high-throughput next-generation sequencing (NGS), provides a comprehensive view of the library's evolution, revealing sequence convergence and mutational hotspots critical for target binding. The kinetics of the selected interactions are characterized using advanced assays like Surface Plasmon Resonance (SPR), which offers real-time measurements of binding events, including association (k_on) and dissociation (k_off) rates. These measurements are crucial for determining the equilibrium dissociation constant (K_D), which quantifies the affinity of the interaction. Beyond kinetics, structural analysis tools like X-ray crystallography and Cryo-Electron Microscopy (Cryo-EM) are employed to resolve the three-dimensional binding interface at atomic resolution, providing insights into the molecular determinants of specificity and guiding rational design efforts.
Phage display’s impact on drug discovery has been profound, particularly in the development of monoclonal antibodies and peptide-based therapeutics. The technology’s ability to generate and screen vast libraries of single-chain variable fragments (scFvs), Fab fragments, and full-length antibodies has accelerated the development of biologics, enabling the precise targeting of proteins involved in diseases like cancer, autoimmune disorders, and infectious diseases. Advanced affinity maturation techniques, such as DNA shuffling, allow for the systematic combination of beneficial mutations, while error-prone PCR introduces controlled variability to refine and optimize binding characteristics. These methods not only improve affinity but also enhance the stability, solubility, and pharmacokinetics of candidate molecules, increasing their potential as therapeutics.
In diagnostics, phage display has enabled the development of highly sensitive and specific detection reagents. Antibodies identified through phage display serve as the foundation for ELISA assays, lateral flow tests, and other diagnostic platforms that rely on the precise identification of disease biomarkers. Epitope mapping, facilitated by phage display, pinpoints the specific regions of an antigen that are recognized by the immune system, providing valuable information for the design of vaccines and diagnostic tools. In vaccine development, phage display has been instrumental in identifying immunogenic epitopes that can elicit protective immune responses. Researchers employ both linear and conformational epitope mapping to discover sequences that trigger neutralizing antibodies, guiding the creation of subunit vaccines and contributing to the development of next-generation vaccines targeting a broad range of pathogens.
Despite its successes, phage display is not without limitations. Constructing highly diverse and representative libraries is challenging, given the inherent constraints of transformation efficiency, codon bias, and DNA stability during amplification. Library diversity is critical, as insufficient variation can lead to early convergence on suboptimal candidates, limiting the discovery potential. Additionally, nonspecific binding remains a persistent problem, often addressed by optimizing blocking agents, incorporating negative selection steps, and implementing stringent washing protocols. Different phage systems present unique technical obstacles—M13’s sensitivity to structural alterations limits its use for larger or complex proteins, while T7’s rapid lytic cycle can lead to the loss of low-abundance clones during amplification. Solutions, like the use of hyperphage systems for improved pIII display efficiency and the implementation of gentle elution techniques, demonstrate the adaptability of phage display technology in addressing these challenges.
Innovations continue to expand the capabilities of phage display, integrating emerging technologies to overcome traditional limitations. In vitro display alternatives, such as ribosome and mRNA display, bypass the need for living cells, facilitating the construction of ultra-large libraries with diversity levels exceeding 10¹³–10¹⁵ variants. These cell-free systems enable rapid selections and avoid the transformation bottlenecks inherent to bacterial systems, although they require careful handling due to the instability of ribosomal complexes and RNA degradation. The incorporation of computational modeling has further enhanced phage display by providing in silico predictions of protein-protein interactions, guiding the design of libraries with targeted mutations, and accelerating the optimization process. These tools allow for the rational design of binders, focusing experimental efforts on high-probability candidates and reducing the trial-and-error aspect of traditional methods.
High-throughput automation and deep sequencing have revolutionized the scale and efficiency of phage display. Robotic liquid handlers and automated screening platforms streamline the biopanning process, enabling the parallel screening of thousands of candidates and reducing human error. Deep sequencing provides a granular view of the selection process, identifying rare but potentially valuable clones that might be overlooked in traditional methods. This detailed tracking allows for real-time adjustments to selection protocols, increasing the likelihood of identifying optimal candidates. The ability to integrate these advanced technologies with phage display has led to more precise and reliable outcomes, cementing its role as a foundational tool in molecular biology.
The future of phage display lies in its continued evolution and adaptation to meet the growing demands of biotechnology, synthetic biology, and personalized medicine. Efforts to improve phage stability, display capacity, and selection efficiency are ongoing, driven by innovations in vector design, display proteins, and phagemid engineering. The convergence of phage display with other display technologies—such as yeast display, mammalian display, and cell-free systems—promises to create hybrid platforms that combine the best attributes of each system, offering unparalleled versatility and power. The integration of machine learning and artificial intelligence for data analysis is expected to further refine phage display, predicting binding affinities and guiding mutagenesis with increasing accuracy.
Ultimately, phage display is more than just a technique; it is a versatile platform that continues to adapt and evolve, driving scientific discovery and technological innovation. Its ability to identify, optimize, and engineer molecules with high specificity and affinity has not only advanced fundamental research but has also led to practical applications across medicine, diagnostics, industrial biotechnology, and materials science. As phage display technology progresses, its role in solving complex biological problems will only expand, opening new possibilities for understanding the molecular underpinnings of disease, designing next-generation therapeutics, and engineering sophisticated biomolecules. This article has aimed to provide a comprehensive exploration of the intricate technical landscape of phage display, highlighting both the challenges and the innovations that make this technology an indispensable tool for modern molecular biology and a catalyst for future breakthroughs.