Selecting Fluorescent Dyes and Quenchers for Oligonucleotide Labeling – Complete Guide
From spectra to stability: how to choose, pair, and validate labels that work, choices for qPCR, FRET, imaging, and multiplex panels, the why and how behind dye–quencher selection for reliable results
Fluorescent labeling of oligonucleotides is pivotal in biotechnology for applications ranging from real-time PCR (qPCR) assays to Förster resonance energy transfer (FRET) experiments and fluorescence in situ hybridization (FISH). Choosing the right fluorescent dye and quencher is a multi-criterion decision. Researchers must balance optical performance (brightness, photostability, spectral properties) with chemical and practical considerations (pH and thermal stability, solubility, cost, and compatibility with the assay). This article reviews the key selection criteria for fluorescent dyes and quenchers, and discusses how these apply to qPCR probes, FRET pairs, and general oligonucleotide labeling for imaging and detection. We include comparative data tables and references from dye manufacturers and peer-reviewed sources to ensure scientific rigor.
Key Selection Criteria for Fluorescent Dyes
When selecting a fluorescent dye for labeling an oligo, several core properties determine its suitability:
Brightness (Extinction Coefficient × Quantum Yield) – How intense is its fluorescence?
Photostability – Does it resist photobleaching under illumination?
Spectral Characteristics – Excitation and emission wavelengths, Stokes shift, and overlap with other fluorophores.
Environmental Stability – Sensitivity to pH changes, solvent polarity, and temperature (especially for qPCR).
Chemical Structure & Compatibility – Size, charge, and functional groups affecting solubility and conjugation.
Cost and Availability – Practical considerations like dye cost, licensing, and availability in required reactive forms.
Each of these factors is detailed below.
Brightness: Extinction Coefficient and Quantum Yield
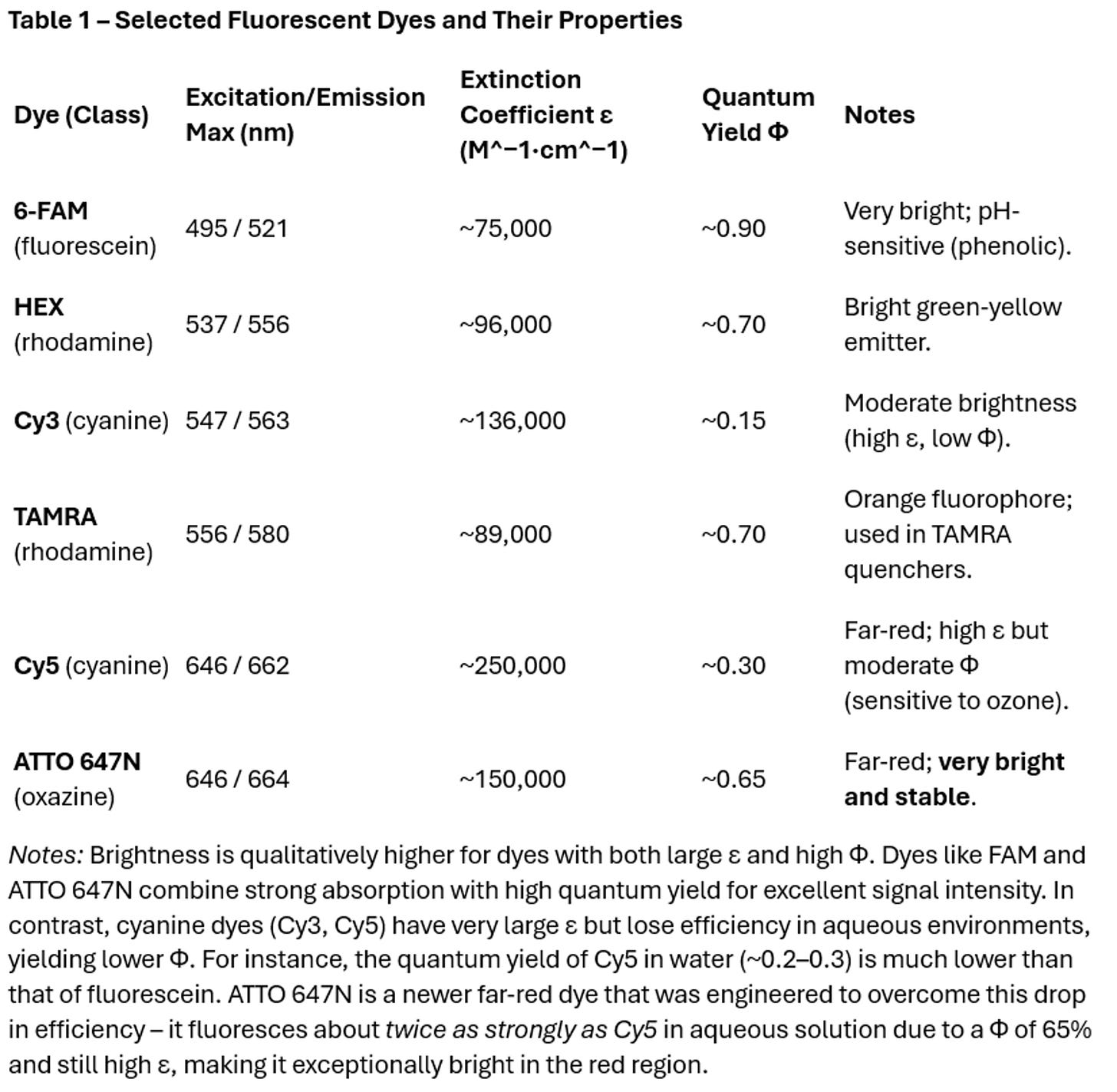
A dye’s brightness is the product of its molar extinction coefficient (ε, how strongly it absorbs light at the excitation wavelength) and its fluorescence quantum yield (Φ, the fraction of absorbed photons re-emitted as fluorescence). In simple terms, brightness measures how much fluorescent signal a dye can produce for a given illumination. A high extinction coefficient and high quantum yield are both desirable. For example, 6-FAM (a fluorescein derivative) has an excitation maximum around 495 nm with ε ≈ 75,000 M^−1cm^−1 and a quantum yield ~0.9, making it very bright. By contrast, Cy3, a popular orange-emitting dye, has a very high ε (~136,000) but a lower quantum yield (~0.15 in typical conditions), resulting in moderate overall brightness. Table 1 compares these values for several common dyes:
Chemical Structure and Spectral Properties of Fluorescent Dyes
The photophysical performance of a fluorescent dye—its absorption and emission characteristics, quantum yield, photostability, and environmental sensitivity—is intrinsically governed by its chemical structure. Fluorophores used in oligonucleotide labeling broadly fall into several distinct structural categories, including xanthene dyes, cyanine dyes, rhodamines, phenoxazines/oxazines, and more recently, rigidized or PEGylated derivatives. Each scaffold contributes uniquely to the dye's behavior in aqueous biological environments and its compatibility with labeling chemistries.
1. Xanthene-Based Dyes
Xanthene dyes such as fluorescein and tetramethylrhodamine (TAMRA) are among the earliest fluorophores used in nucleic acid chemistry. Their tricyclic core structure enables high fluorescence quantum yields (>0.8), with emission in the green-orange range (500–580 nm). However, xanthenes are known to exhibit pronounced pH dependence due to their ionizable phenolic groups.
For example, fluorescein displays a marked drop in fluorescence below pH 6.5, a phenomenon well-characterized in the study by Martin & Lindqvist (1975), where the authors attribute the pH sensitivity to changes in the anionic equilibrium of the fluorophore. This restricts its use in acidic environments or applications involving buffer fluctuations, such as isothermal amplification reactions.
2. Cyanine Dyes
Cyanine dyes, including Cy3, Cy5, DY-547, DY-681, and AZDye 594, feature two nitrogen-containing heterocycles bridged by a polymethine chain. The degree of π-conjugation—dictated by the length and planarity of the chain—determines the position of the dye’s absorption and emission maxima. Longer chains shift fluorescence emission into the red and near-infrared (NIR) regions.
Cyanines are favored for their high molar extinction coefficients (often >100,000 M⁻¹cm⁻¹) and tunable spectra, making them ideal for FRET and multiplexing. However, their spectral flexibility comes with drawbacks. Many unmodified cyanines are prone to photobleaching, susceptible to ozone degradation, and can exhibit aggregation in aqueous media due to their hydrophobic character. Modern cyanine dyes, such as those from Dyomics (e.g., DY-647P1, DY-634), incorporate sulfonation or PEGylation to enhance water solubility and reduce nonspecific binding, while maintaining photostability.
Additionally, rigidized cyanines (e.g., Alexa Fluor 647, ATTO 647N) introduce structural constraints to reduce conformational flexibility of the polymethine chain. This results in higher quantum yields and enhanced resistance to photo-induced degradation.
3. Rhodamine and Rhodamine-Derived Dyes
Rhodamines, including Rhodamine B, Rhodamine 6G, and Alexa Fluor analogs like Alexa 546 or ATTO 550, share structural similarities with xanthenes but incorporate quaternary amines, enhancing photostability and pH robustness. Their emission spans the orange to red region (~550–610 nm), with high brightness and favorable aqueous solubility.
These dyes are typically cationic at physiological pH, facilitating efficient conjugation to negatively charged oligonucleotides or proteins. Importantly, rhodamines often exhibit lower environmental sensitivity, maintaining fluorescence across a wide range of pH, ionic strength, and buffer compositions. As a result, they are commonly employed in qPCR probes, where fluorescence stability during thermal cycling is essential.
4. Phenoxazines and Oxazines
Phenoxazine- and oxazine-based dyes such as ATTO 655, ATTO 680, and Nile Blue derivatives are structurally more complex, incorporating additional heteroatoms into extended aromatic ring systems. These modifications shift absorption and emission into the far-red and NIR regions (650–750 nm) and confer large Stokes shifts, which are advantageous for minimizing spectral crosstalk in multiplexed assays.
Their chemical structure enables strong π-π stacking and interaction with nucleic acid bases, which can be either a feature or a limitation depending on the application. However, these dyes often demonstrate excellent photostability and resistance to pH and temperature extremes, making them suitable for advanced imaging and in vivo diagnostics.
Impact of Structural Modifications on Fluorophore Performance
To overcome limitations associated with native fluorophore scaffolds, many commercial dyes undergo chemical modification aimed at improving their biocompatibility, stability, solubility, and conjugation specificity. These structural enhancements are particularly crucial when working in complex biological systems where dyes encounter varying pH, ionic strength, nucleic acid interactions, and reactive functional groups.
1. PEGylation: Enhancing Solubility and Reducing Aggregation
Polyethylene glycol (PEG) chains are frequently appended to fluorophores to increase their hydrophilicity, reduce hydrophobic aggregation, and improve aqueous solubility. This is particularly important for long-chain polymethine dyes, such as traditional cyanines, which otherwise tend to stack or self-quench due to hydrophobic interactions.
For instance, DY-680-PEG and related PEGylated dyes from Dyomics are optimized for use in serum-containing media and crowded cellular environments. PEGylation also reduces nonspecific binding to proteins, membranes, or oligonucleotide backbones, thereby improving signal-to-noise ratios and maintaining fluorescence quantum yield.
2. Sulfonation: Charge Modulation and Water Compatibility
The introduction of sulfonate groups (-SO₃⁻) into fluorophore structures serves multiple purposes:
· Increases negative charge, enhancing electrostatic repulsion between dye-labeled biomolecules (reducing aggregation).
· Improves solubility in polar buffers and aqueous systems.
· Prevents hydrophobic interactions with oligonucleotide bases or membrane surfaces.
· This is especially beneficial for cyanine dyes, which can otherwise form non-specific dye-DNA interactions or exhibit background signal due to π-stacking with nucleobases. Sulfonated Cy5 analogs, such as Alexa Fluor 647 and ATTO 647N, are more photostable and spectrally consistent in real-time PCR and smFRET assays.
3. Reactive Functional Groups: NHS Esters and Maleimides
Functional groups such as NHS esters (N-hydroxysuccinimide) and maleimides are added to fluorophores to allow site-specific bioconjugation:
NHS esters react with primary amines, commonly found on lysine residues or amino-modified oligonucleotides. The reaction occurs efficiently at pH 8.3–9.0 and forms a stable amide bond, as detailed in ATTO-TEC’s NHS-Ester application guidelines.
Maleimides react with thiol groups (e.g., from cysteine residues or thiol-modified DNA/RNA) under mild conditions, forming a stable thioether linkage.
These conjugation strategies allow researchers to precisely label oligonucleotides at the 5′ or 3′ end, or at internal positions, without compromising structural integrity or hybridization behavior. The stability of these linkages ensures compatibility with high-temperature cycling (e.g., qPCR) or extended imaging protocols.
4. Structural Rigidification and π-System Locking
Certain dyes incorporate chemical elements that rigidify the polymethine backbone, reducing rotational freedom and non-radiative decay. This results in:
· Improved quantum yield (more photons emitted per excitation)
· Higher photostability (resistance to bleaching over time)
· Narrower emission peaks, enhancing resolution in multiplex detection
For example, Alexa Fluor and ATTO dyes achieve enhanced brightness and lifetime stability through molecular engineering that locks the dye in a planar, rigid configuration. This rigidity suppresses torsional relaxation and stabilizes excited-state energy levels.
Structural modifications, whether via PEGylation, sulfonation, reactive group installation, or core rigidification, enable modern fluorescent dyes to perform reliably in complex biochemical and cellular systems. These enhancements not only extend dye utility across diverse oligonucleotide-based platforms but also provide tunable control over labeling efficiency, background noise, and signal stability.
In practice, a brighter dye enables detection of lower-abundance targets or allows using lower laser power. As a rule, pair bright dyes with low-abundance targets and reserve dimmer dyes for high-abundance targets to ensure all signals fall in a detectable range. It’s also important to consider instrument sensitivity at the dye’s emission wavelength – brightness is only useful if the detector and filters can efficiently capture that fluorescence.
Photostability and Chemical Stability
Photostability is the ability of a fluorophore to resist photobleaching (permanent loss of fluorescence) under illumination. Photobleaching is an irreversible photochemical destruction of the fluorophore, often accelerated by molecular oxygen or other reactive species. Under intense or prolonged excitation (as in confocal microscopy or multi-scan microarray imaging), dyes can rapidly lose signal. For example, fluorescein (FITC) is notoriously prone to photobleaching, which limits its use in long-term imaging. In contrast, modern dyes have been developed for high photostability. The ATTO series dyes are one such family designed to withstand extended irradiation; they remain intact and fluorescent for much longer than traditional dyes under the same conditions. One study shows that ATTO 655 (far-red dye) retains fluorescence far better than Cy5 under continuous light exposure. In practical terms, improved photostability translates to brighter images over time and the ability to collect multiple scans or lengthy time-lapse sequences without severe signal loss.
Closely related is chemical stability, including resistance to environmental degradants. One notable culprit in laboratories is ozone. Atmospheric ozone at even low concentrations (a few ppb) can rapidly degrade certain cyanine dyes like Cy5, causing fluorescence signal loss even in the dark. This is a known issue in microarray experiments where Cy5-labeled targets on slides can fade due to ozone exposure. Dye manufacturers have tackled this by creating more ozone-resistant fluorophores. For instance, ATTO 647N and ATTO 655 are reported to last up to 100× longer than Cy5 or Alexa 647 when exposed to typical lab air ozone levels. Similarly, a novel dye called “HyPer5” was developed to be far more ozone-stable than Cy5, maintaining consistent microarray performance in high-ozone environments. If oligo labels will be used in microarray, outdoor fluorescence imaging, or other settings with potential ozone exposure, choosing an ozone-resistant dye is critical to avoid signal quenching before detection.
In summary, dyes with enhanced photostability and chemical stability (e.g. ATTO dyes, Dyomics DY-series, or specially stabilized dyes) are preferred for demanding applications. Their robust performance under light and oxidative stress improves sensitivity and data reliability. Conversely, if using classic dyes like fluorescein or cyanines, one may need to add anti-fade agents or take precautions (e.g. minimize light exposure, control atmosphere during scans) to mitigate degradation.
Spectral Properties: Excitation, Emission, Stokes Shift and Crosstalk
The spectral profile of a dye – its excitation and emission wavelengths and the separation between them – determines how it fits into an experiment’s optical setup. Key considerations include:
Excitation Wavelength: The dye should absorb strongly at a wavelength your instrument can provide (e.g. laser or LED line). Ideally, the excitation maximum of the dye aligns with a available light source. If not exact, a nearby match can work, but absorption drops off away from the peak. A practical tip is if no dye matches the laser exactly, choose one with an absorption slightly longer (red-shifted) than the laser line; though ε will be a bit lower, the larger Stokes shift can ease emission separation. Moreover, redder excitation (>550–600 nm) is often preferable for biological samples because it avoids auto-fluorescence from cells/tissues and reduces photodamage.
Emission Wavelength: The emission should fall within a detector’s range and align with available filter sets. Emission spectrum overlap with detection filters affects how much signal is captured versus lost. For example, a dye emitting at 520 nm (like FAM) pairs well with a “green” filter set, whereas a 670 nm emitter (Cy5/ATTO 647N) needs a far-red detector. The emission also must be separable from other dyes in multiplex experiments (discussed below).
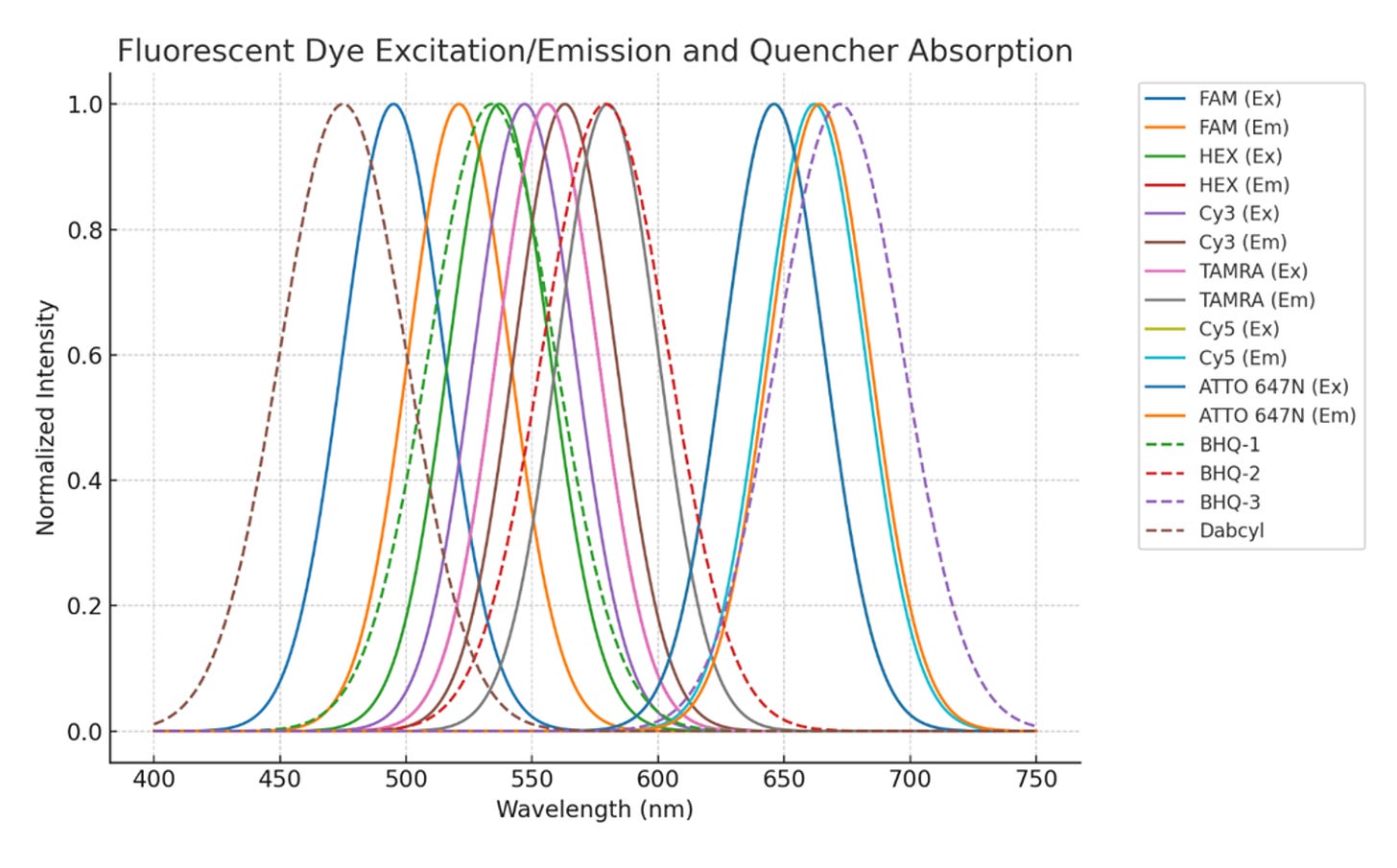
Stokes Shift: The Stokes shift is the gap between the excitation and emission peaks. A larger Stokes shift (i.e. the dye emits at a much longer wavelength than it was excited) is highly beneficial. Large Stokes shifts minimize overlap between excitation light and emission, improving signal-to-noise by making it easier to filter out excitation scatter. They also reduce self-quenching phenomena like re-absorption: emitted photons are less likely to be reabsorbed by neighboring dye molecules if there’s a big spectral gap. Dyes with very small Stokes shifts can suffer from re-excitation or inner-filter effects in concentrated samples. For multicolor experiments, large Stokes shift dyes help avoid crosstalk – they allow using fewer detection channels or avoiding bleed-through between channels. For instance, a dye with a 165 nm Stokes shift could be excited in the UV and emit in the visible, far from the excitation source. ATTO-TEC’s “LS” dyes (e.g. ATTO 430LS, ATTO 490LS) are examples with exceptionally large Stokes shifts (~150 nm) specifically to address channel cross-talk. These dyes proved ideal for multiplex imaging where conventional fluorophores would spectrally overlap. One trade-off, however, is that historically many large-Stokes-shift dyes (often based on coumarin structures) tended to have lower brightness or photostability. Recent advances (such as Dyomics’ “MegaStokes” coumarin dyes) have produced large-Stokes fluorophores that are much brighter and more photostable than older coumarins. Still, when using such dyes, consult data to ensure their brightness is sufficient, as not all large-Stokes dyes are created equal.
Spectral Overlap and Crosstalk: In multiplex assays (multiple dyes in one sample), it’s crucial that each dye’s emission is distinct or can be unmixed. Crosstalk refers to bleed-through of one dye’s fluorescence into the detection channel of another. To minimize this, choose dyes with well-separated emission spectra or use filters that tightly restrict bandwidth. For example, a common 4-color qPCR set might use FAM (~520 nm), HEX (~556 nm), ROX (~605 nm), and Cy5 (~670 nm) – each roughly 40–60 nm apart so that with proper filter sets, crosstalk is limited. If emissions are too close, signals will overlap (requiring spectral compensation or confounding analysis). Using dyes that have narrow emission peaks or far apart peaks simplifies detection. Additionally, pay attention to excitation overlap: if two dyes are excited by the same light source, one must ensure that the detection of each is specific (for instance, in FRET experiments, the donor is excited and the acceptor emits; the acceptor may also be directly excitable by the donor’s excitation wavelength – this direct excitation must be accounted for or minimized by design). Overall, a spectral compatibility table or emission/excitation chart of candidate dyes should be consulted to ensure a clean separation of signals in your setup.
Environmental Factors: pH and Thermal Stability
The performance of many fluorophores is environment-dependent. Two important factors for oligonucleotide applications are pH sensitivity and thermal stability:
pH Dependence: Some dyes change their fluorescence intensity or spectra with pH. A classic example is fluorescein (and its derivatives like FAM), which is largely deprotonated and highly fluorescent at pH ≥8 but becomes protonated and much less fluorescent at pH ≤6 (its phenolic groups have pK_a ~6.4). In nucleic acid applications, buffers are typically near neutral pH 7–8, so fluorescein tags work well (and indeed FAM is ubiquitous for 5′-labeling). However, if an experiment involves acidic conditions (e.g. inside endosomes or certain FISH protocols) or a broad pH range, a pH-stable dye is preferable. Many newer dyes are engineered to be insensitive across a wide pH range. For instance, most ATTO dyes have optical properties independent of pH from ~2 to 11, meaning you can trust their brightness in virtually any biologically relevant environment. Similarly, rhodamine-based dyes (e.g. TAMRA, Alexa 546) tend to be less pH-sensitive than fluoresceins. Always check the dye’s spec sheet or literature for phrases like “pH-independent fluorescence”. If none are given, assume some pH effect might occur and test the dye at your working pH. In summary, for assays outside neutral pH, choose known pH-stable fluorophores (or measure fluorescence vs. pH in a pilot test).
Thermal Stability: Oligonucleotide probes may be subjected to high temperatures, especially in PCR-based assays. During qPCR, for example, probes experience repeated cycling to 95 °C for denaturation, and they must survive intact (both chemically and fluorescence-wise) for many cycles. While most dyes can endure brief high temps, some are more prone to degradation or deconjugation at elevated temperatures. Cyanine dyes can degrade under prolonged heat, and certain quencher molecules (like early Dark Quenchers) had stability issues in extreme thermal conditions. In contrast, ATTO dyes are noted for increased thermal stability, attributed to their rigid, robust structures. Indeed, ATTO 647N is highlighted as having high thermal stability, making it suitable for demanding applications like single-molecule detection and super-resolution microscopy that might involve heating steps. For qPCR probes, practical experience has shown that dyes like FAM, HEX, Cy5, etc., generally survive PCR cycling well, but newer alternatives (ATTO, CF dyes, etc.) can offer even greater stability if needed (and may come into play for isothermal amplification at elevated temperatures or long exposure to 95 °C in certain protocols). If a dye/quencher pair will be exposed to high heat repeatedly, verify that both labels are described as “thermostable” or recommended for PCR use. Sometimes manufacturers explicitly label certain dyes as suitable for qPCR probes. A probe failing mid-run due to dye degradation would result in a loss of fluorescence signal, falsely appearing as if target amplification dropped. Thus, thermal robustness is a must for quantitative PCR probes or any assay with heat steps.
Dye Chemistry, Solubility and Compatibility
Not all fluorophores are created equal in terms of chemical makeup. The structure and chemistry of a dye influence how it can be attached to an oligonucleotide and how it behaves in biological solutions. Key points include:
Reactive Form and Conjugation: To label an oligonucleotide, the dye must be available in a suitable reactive form (e.g. a phosphoramidite for direct DNA synthesis incorporation, or an NHS ester, maleimide, azide, etc., for post-synthesis conjugation). Ensure the dye you select is sold in the form you need. Most common dyes are, but exotic fluorophores might not be easily available for DNA labeling. Additionally, some dyes survive certain chemistries better – for example, DNA synthesis requires the dye (if a phosphoramidite) to withstand coupling and ammonia deprotection. Fluorescein and Cy dyes handle this, as do ATTO phosphoramidites (many ATTO dyes are offered as phosphoramidites for direct 5′ labeling during synthesis). Always use a dye derivative specifically designed for your labeling method to avoid degradation or poor coupling yields.
Hydrophobicity and Solubility: Dyes vary from highly water-soluble to very hydrophobic. Cyanine dyes often have sulfonate groups to improve solubility (e.g. Cy3, Cy5 are sulfonated and thus fairly hydrophilic). Many classic fluorophores (fluorescein, TAMRA) are moderately hydrophilic anions. On the other hand, some older dyes and certain dark quenchers (like Dabcyl) are quite hydrophobic. A hydrophobic dye on an oligo can cause aggregation or nonspecific binding to surfaces and proteins. For instance, Dabcyl quenchers on probes sometimes led to solubility issues in aqueous assays. To address this, newer quencher designs and dye analogues incorporate charged or polar substituents or use polyethylene glycol (PEG) spacers to increase hydrophilicity. Dyomics has a line of PEGylated dyes – their latest catalog highlights PEGylated fluorescent labels with reduced nonspecific binding. Such modifications improve water solubility and prevent dyes from sticking to reaction tubes or cell components non-specifically. If your assay involves complex mixtures (serum, cell lysates) or if you observe unexpected probe aggregation, consider using or switching to a more hydrophilic dye variant. In summary, match the dye’s polarity to your application: highly hydrophobic dyes may be fine for immobilized assays (binding to a surface can even be aided by hydrophobicity), but for in-solution or in vivo applications, a water-soluble dye yields cleaner results.
Size and Linker Length: The physical size of a dye molecule and the length of the linker attaching it to the oligo can impact performance. Very bulky dyes attached directly at an oligo terminus might hinder hybridization by steric hindrance, or, in the case of certain FRET constructs, might affect the relative orientation of donor and acceptor. Sometimes a short linker (e.g. a 6-carbon spacer or a triethyleneglycol spacer) is used to distance the dye from the oligonucleotide to reduce interference. Additionally, bulky or multicyclic dyes can have slower rotational diffusion, which might influence fluorescence anisotropy or energy transfer efficiency (κ^2 factor in FRET). In general, most single dyes on small oligos do not dramatically alter hybridization, but it is something to consider if adding multiple dyes or a very large label (some labels like certain enzyme substrates or intercalators are bigger than the oligo itself!). When in doubt, consult literature or the dye supplier for any noted effects of the dye on oligo hybridization or function. Many suppliers provide notes if a dye is known to quench when in duplex or if a longer linker is recommended for certain applications.
Rigid vs. Flexible Structure: The molecular rigidity of the dye influences its photophysics. As noted in an LGC oligo modifications blog, ATTO dyes have a relatively rigid polycyclic structure (often derivatives of coumarin, rhodamine, etc.), which prevents formation of multiple isomers and yields consistent optical properties regardless of solvent or temperature. In contrast, polymethine cyanines are more flexible and can adopt different conformations, sometimes leading to environment-sensitive behavior (e.g. Cy5 can form cis/trans isomers or stack with neighboring bases in DNA, causing fluorescence variability). Thus, rigid dyes are often more predictable and stable in their fluorescence output. This can be an advantage in quantitative assays: ATTO dyes, for example, are noted to perform nearly independently of solvent polarity and temperature. If your experiment demands high quantitative accuracy, choosing a dye known for consistency (rigid structure, minimal environmental quenching) can improve reproducibility.
Cost and Licensing: Lastly, a practical note – some dyes are proprietary or more expensive, which might influence selection especially for large-scale projects. Dyes like Cy3/Cy5, Alexa Fluors, or certain proprietary quenchers may have licensing fees or higher costs. Alternatives from independent manufacturers (e.g. ATTO-TEC, Dyomics, Biotium’s CF® dyes) often provide similar performance without encumbered IP or at lower cost. For instance, ATTO dyes have been promoted as high-performance substitutes for Alexa or Cyanine dyes in qPCR and digital PCR probes. When budget is a concern, it is reasonable to compare the performance and cost of an alternative dye. Peer-reviewed literature or vendor application notes can be helpful to ensure the alternative is truly comparable. In any case, cost should be weighed after technical suitability – a cheap dye that fails in your assay is far more costly in the end. But when multiple dyes meet the technical needs, cost and supplier reliability can be deciding factors.
Quencher Selection Criteria
Fluorescent probes in qPCR and many FRET-based assays use a quencher paired with a fluorescent reporter. Quenchers are molecules that absorb the fluorescence from the reporter (by FRET or contact quenching) and dissipate the energy as heat, thereby suppressing fluorescence until an assay endpoint is achieved (e.g. probe cleavage or conformational change separates reporter and quencher). The ideal quencher has these characteristics:
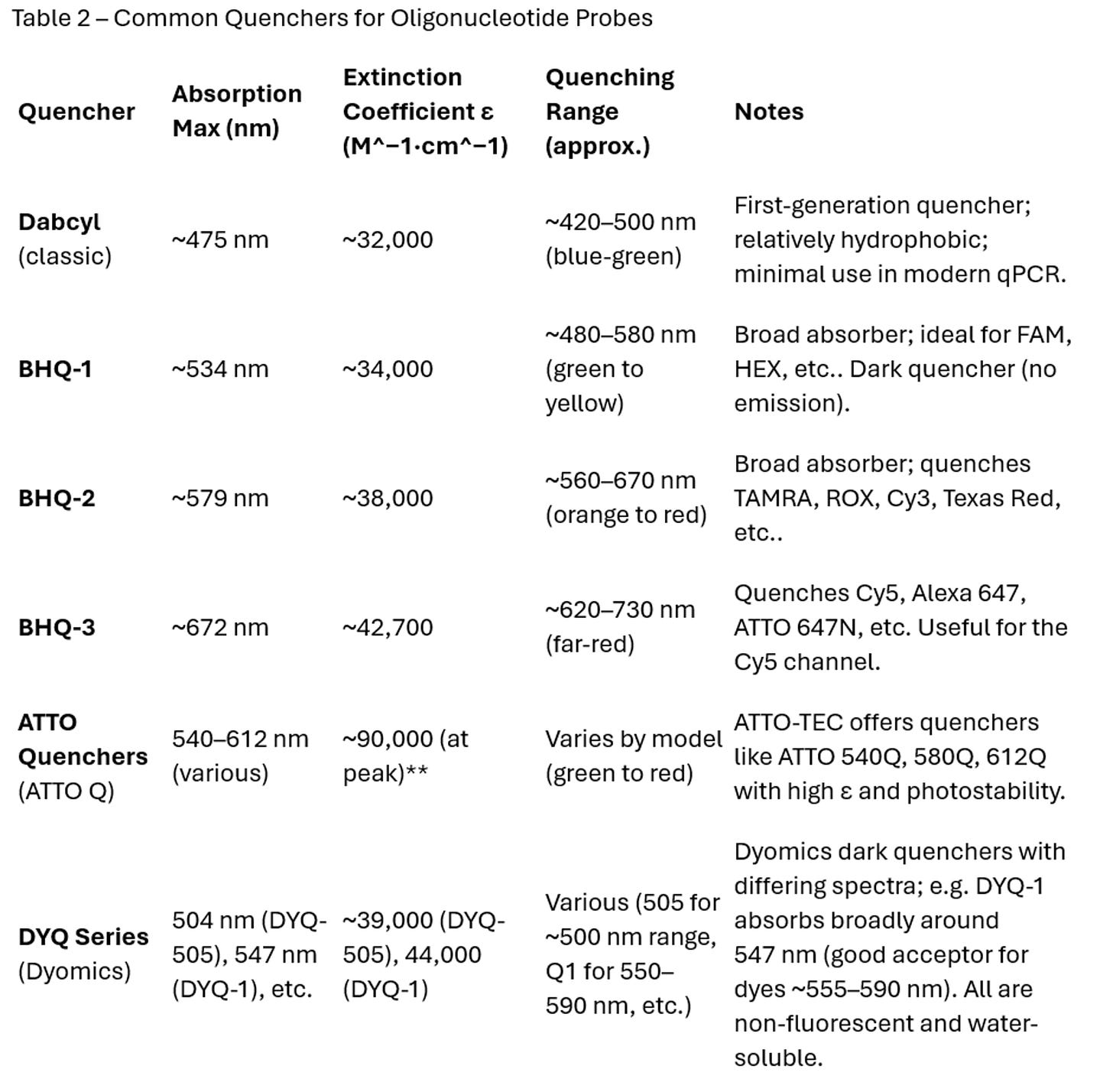
Strong Broad Absorption in the Relevant Range: A quencher must absorb light where the reporter emits. Unlike fluorophores, dark quenchers do not re-emit light (quantum yield ~0), they simply convert it to heat. The absorption spectrum of a quencher should significantly overlap the emission spectrum of the dye to ensure efficient energy transfer. Modern quenchers like the Black Hole Quencher® (BHQ) series are designed with very broad absorption bands covering entire swaths of the spectrum. For example, BHQ-1 has maximum absorbance around 534 nm and effectively quenches fluorophores emitting from ~480–580 nm. BHQ-2 (λ_max ~579 nm) covers roughly 560–670 nm, suitable for quenching orange-red dyes, and BHQ-3 (λ_max ~672 nm) covers ~620–730 nm for quenching far-red/NIR dyes. These quenchers have extinction coefficients on the order of 30,000–40,000 M^−1cm^−1 at their peaks – high enough to efficiently soak up the emitted photons of typical reporters. Table 2 summarizes common quencher properties:
Spectral overlap plot showing excitation (Ex) and emission (Em) profiles of common fluorescent dyes (solid lines) and absorption spectra of quenchers (dashed lines). Optimal dye–quencher pairing occurs when the quencher’s absorption curve significantly overlaps the dye’s emission curve, enabling efficient energy transfer and minimizing background fluorescence. This figure can guide the selection of reporter–quencher combinations for qPCR, FRET, hybridization probes, and multiplexed assays.
Tips for Use:
For qPCR, select bright, thermally stable dyes (e.g., FAM, ATTO 647N) and match them to quenchers whose absorption range fully covers the emission spectrum.
For multiplex assays, choose dyes with well-separated emission peaks and low spectral crosstalk; refer to vendor spectral separation tables.
Align quencher absorption maxima at or slightly beyond the dye emission maximum for optimal quenching.
Use double-quenched probes to reduce baseline fluorescence in highly sensitive qPCR assays.
For tissue imaging, far-red or NIR dyes (e.g., ATTO 647N, Cy5) minimize autofluorescence and increase penetration depth.
Consider photostability: ATTO and Alexa Fluor series generally outperform FITC or traditional cyanines in prolonged illumination.
For ozone-prone environments (e.g., microarrays), avoid Cy5 and use ozone-stable substitutes like ATTO 647N.
For FRET assays, ensure donor–acceptor distance matches the Förster radius (R₀) and donor emission significantly overlaps acceptor absorption.
For in-solution or in vivo applications, hydrophilic or PEGylated dyes improve solubility and reduce aggregation.
For probes requiring extreme pH or temperature stability (e.g., isothermal amplification), select pH-independent and thermally resilient dyes.
Use large Stokes shift dyes (e.g., MegaStokes™) to minimize reabsorption and improve multiplexing performance.
When labeling short oligos, avoid bulky or hydrophobic dyes that could hinder hybridization efficiency.
Always check laser line compatibility of your instrument with dye excitation maxima.
For flow cytometry, match dyes to cytometer laser/filter sets and avoid emission overlaps that cannot be compensated.
When comparing cost, factor in extinction coefficient and quantum yield to assess brightness-per-dollar.
Use matched dye–quencher pairs from the same manufacturer for predictable spectral behavior and lot consistency.
Protect dye-labeled probes from light during storage and transport to prevent photobleaching before use.
Use glycerol-based or sugar-based stabilizers for long-term probe storage at low temperatures.
Do’s:
Do validate dye–quencher performance in your exact assay setup before scaling up.
Do confirm that your detection system’s filters and lasers align with dye properties.
Do compare both ε and Φ values when selecting dyes for brightness.
Do consider the chemical reactivity (NHS, maleimide, phosphoramidite) when planning conjugations.
Do store probes in amber vials or wrap in foil to minimize light exposure.
Don’ts:
Don’t choose dyes solely by popularity—fit to your assay’s conditions is more important.
Don’t use ozone-sensitive dyes in unprotected long-term storage environments.
Don’t assume spectral compatibility without checking actual overlap curves.
Don’t reuse qPCR dye–quencher combinations for imaging without testing photostability under illumination.
Don’t mix dyes with incompatible chemical linkers or solvent requirements in the same probe batch.
Photostability and Environmental Stability
How dyes differ in their ability to resist photobleaching and ozone damage? why this matters for prolonged assays or surface-bound applications like microarrays.
Photostability
Environmental degradation factors (light, ozone)
ATTO 647N, FITC, Cy5 comparison
Brightness vs. Photostability of Common Fluorescent Dyes (Expanded)
Scatter plot showing calculated dye brightness (molar extinction coefficient × quantum yield) versus relative photostability (0–1 scale) for a broad range of commonly used dyes. Higher values on both axes indicate superior optical performance and resistance to photobleaching.
Tips for selection:
For microscopy or long imaging sessions, choose dyes in the upper-right quadrant (e.g., ATTO 565, ATTO 647N).
For high-sensitivity qPCR, prioritize brightness for detection at low copy numbers (FAM, Alexa 488) while ensuring adequate stability.
When multiplexing, select dyes with both high brightness and distinct spectra.
Do’s:Do check laser/filter compatibility before selecting a dye.
Do balance brightness with stability for the intended application.
Don’ts:Don’t rely solely on brightness if the assay involves prolonged illumination—low stability dyes will fade mid-run.
Don’t assume the same dye will behave identically across platforms; validate in situ.
Spectral Properties and Stokes Shift
Excitation/emission spectra, crosstalk, and the benefit of large Stokes shifts. Connect to multi-color assay compatibility.
Define Stokes shift
Benefits of large shifts for multiplexing
Reference to ATTO LS dyes
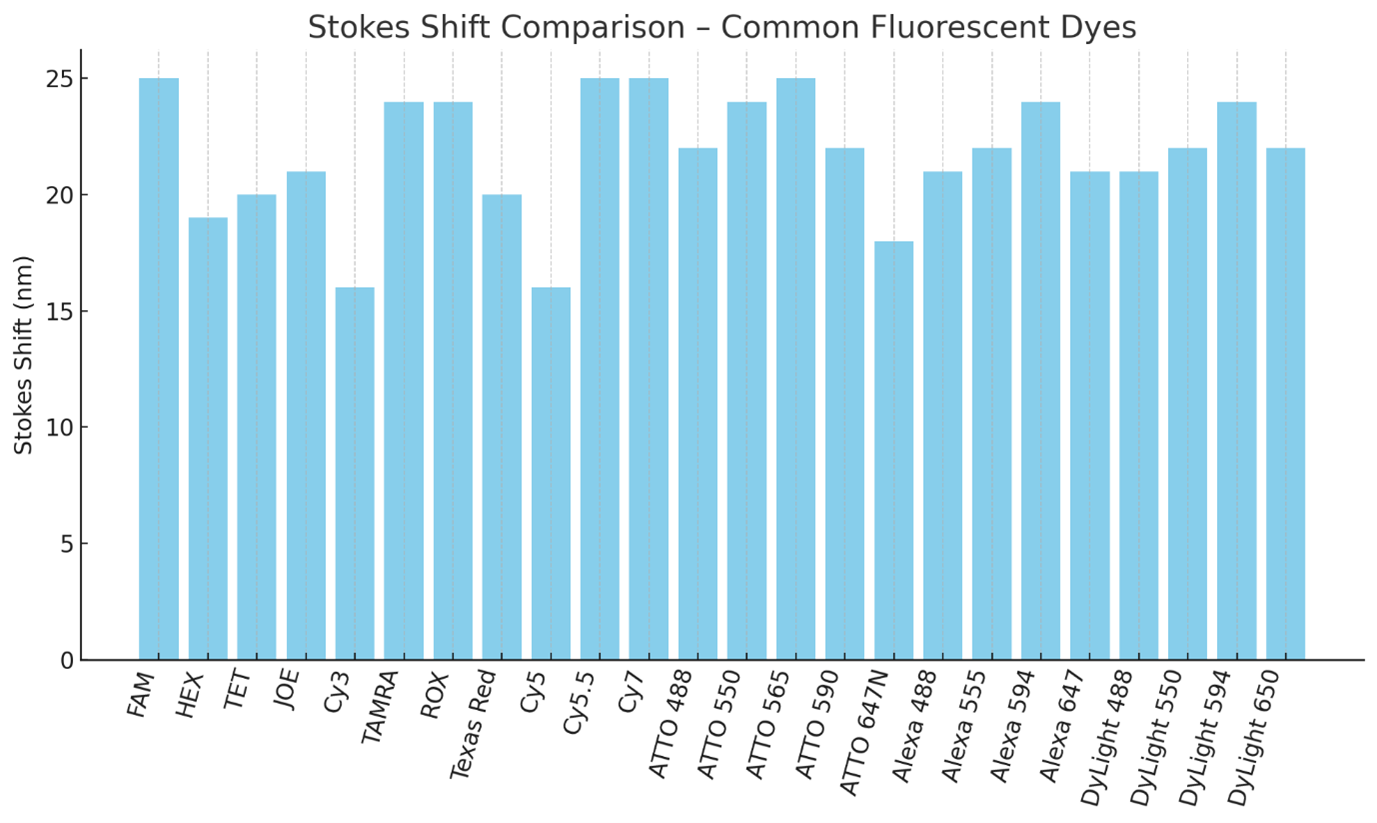
Stokes Shift Comparison – Common Fluorescent Dyes
Bar chart comparing the Stokes shift (difference between excitation and emission maxima) of a wide set of dyes. Larger shifts reduce spectral overlap and reabsorption, improving multiplex assay performance.
Tips for selection:
Use dyes with ≥20 nm Stokes shift when designing multi-color panels.
Large Stokes shift dyes (e.g., ATTO 565, Cy5.5) are especially useful in high-plex qPCR and imaging where filter bleed-through is a concern.
Do’s:Do combine large Stokes shift dyes with narrow-band filters for optimal separation.
Do use vendor spectral plots to verify separation from other fluorophores.
Don’ts:Don’t rely on large shifts alone—also check for brightness and stability.
Don’t mix dyes with overlapping emission tails in multiplex setups unless you have strong compensation capability.
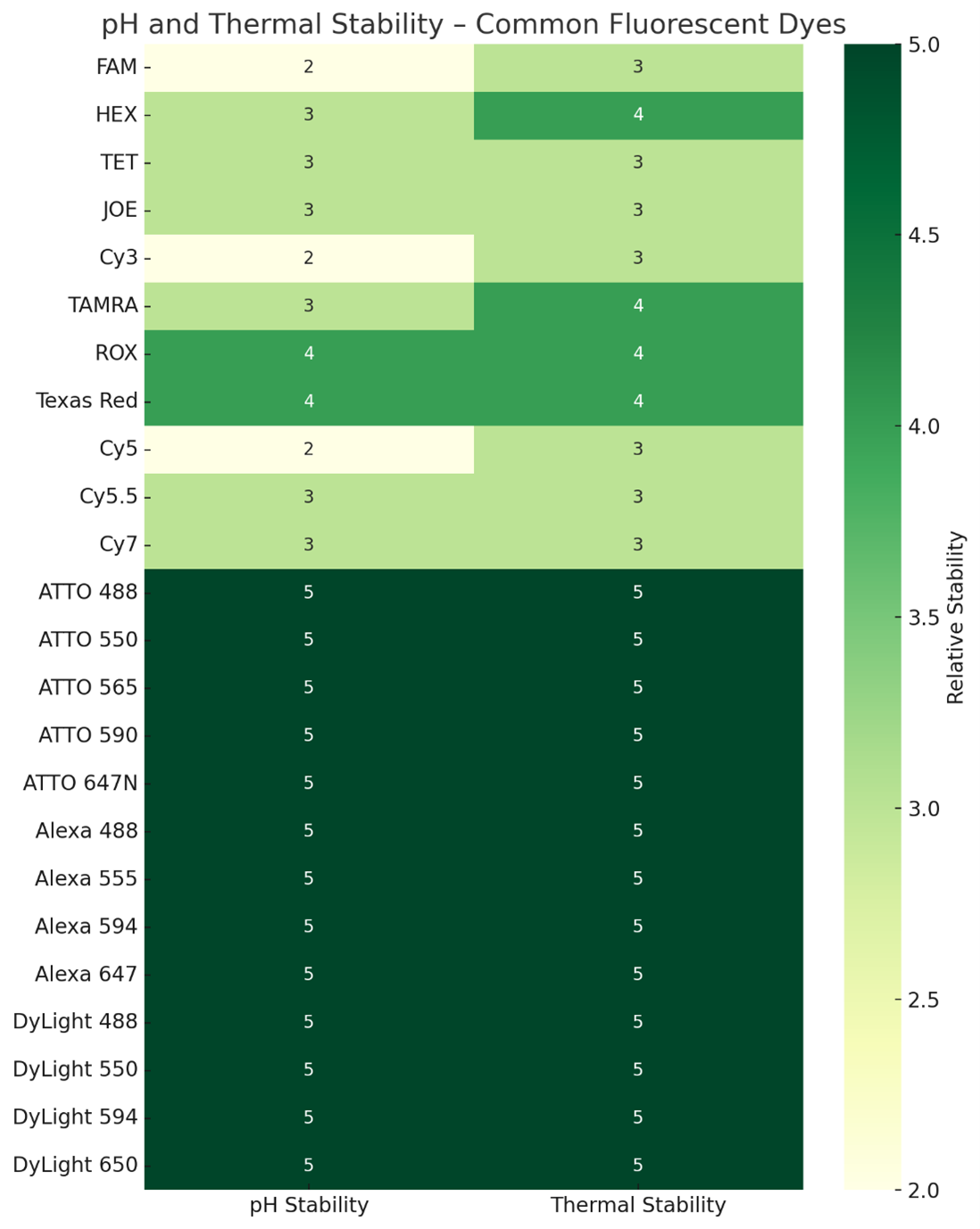
pH and Thermal Stability
How pH and temperature affect dye performance, especially in thermal cycling (e.g., PCR). Discuss dye degradation or signal variation due to pH shifts.
pH-sensitive vs. pH-stable dyes
Heat-resistance in PCR assays
Example: Fluorescein vs. ATTO dyes
pH and Thermal Stability of Common Fluorescent Dyes
Heatmap showing relative stability scores (1–5) for pH and thermal tolerance. Higher numbers indicate greater resistance to signal loss under acidic/alkaline conditions or high temperatures.
Tips for selection:
For qPCR, isothermal amplification, and microarray hybridizations, choose dyes with thermal stability ≥4.
For live-cell imaging in acidic compartments, prioritize pH stability ≥4 (e.g., ATTO 647N, Alexa 647).
Do’s:Do match stability properties to your assay environment.
Do consider pH-stable dyes for experiments involving buffers outside neutral pH.
Don’ts:Don’t use pH-sensitive dyes like FAM for acidic organelle imaging.
Don’t overlook thermal tolerance if probes are exposed to repeated heating cycles.
Dye–Quencher Pairing Matrix – Common Fluorescent Dyes
Heatmap showing spectral compatibility scores between dyes (rows) and quenchers (columns). Green = optimal, yellow = acceptable, red = poor pairing. Scores reflect spectral overlap between dye emission and quencher absorption ranges.
Tips for selection:
Match dyes to quenchers with maximal overlap in emission/absorption to ensure efficient quenching (e.g., BHQ-1 for green-yellow dyes, BHQ-2 for orange-red, BHQ-3 for far-red/NIR).
For multiplex qPCR, choose quenchers with minimal bleed-through into other channels.
Do’s:Do validate quencher efficiency empirically under your assay conditions.
Do select dark quenchers for low-background applications.
Don’ts:Don’t pair dyes with quenchers that have little or no spectral overlap—this leads to high background.
Don’t mix quencher chemistries that differ in solubility or reactivity with your oligo backbone.
Conclusions
Modern quenchers are typically “dark”, meaning they have essentially no fluorescence of their own (quantum yield ~0). This is important to keep background low. Early quenchers like TAMRA or QSY7 were fluorescent acceptors – they absorbed donor emission and re-emitted at a different color. While those can work (and are used in some FRET probe designs), a dark quencher is generally preferable for applications like qPCR because it yields no extra signal to filter out. The BHQ series, Eclipse®, Iowa Black®, ATTO Q, etc., all are dark quenchers. They differ mainly in their absorption profiles and chemical structures. BHQs, for instance, are a set of substituted phenyltriazole compounds with broad absorbance; ATTO’s quenchers (540Q, 580Q, 612Q) are azo-dye based and also claim high photostability. Dyomics’ DYQ quenchers similarly cover key wavelength ranges and are advertised as polar and water-soluble (e.g. DYQ-505 is explicitly described as a polar, water-soluble dark quencher).
Photostability of Quenchers: Quenchers too should be photostable. If a quencher molecule bleaches or breaks down, it may no longer absorb the reporter’s emission, leading to loss of quenching (and hence a creeping up of background fluorescence). In qPCR probes, for example, a bleached quencher can cause higher baseline fluorescence or premature signal. The ATTO and BHQ quenchers are designed to be highly photostable, often more so than the reporters. This ensures the quencher outlives the experiment’s duration. Generally, check that the quencher is recommended for the intended application (most suppliers will indicate if a quencher is suitable for prolonged exposure or not).
Spectral Match to Reporter: When picking a reporter–quencher pair, follow the guidance of spectral overlap. Typically, for common fluorophores: FAM and other fluoresceins (emitting ~520 nm) use BHQ-1 or equivalent; HEX/VIC/JOE (~550 nm) also use BHQ-1 (since it covers up to ~580 nm); TAMRA/ROX (580–610 nm) often use BHQ-2; Cy5 (670 nm) or similar far-red dyes use BHQ-2 or BHQ-3 (BHQ-3 is better centered for >650 nm). Many oligo vendors pre-set these pairings as they are well-tested. If designing a custom probe, refer to absorption spectra: ensure the quencher’s absorbance at the dye’s emission peak is high. It doesn’t have to overlap completely with the emission maximum, but significant overlap is needed for efficient FRET. Also consider the distance between dye and quencher on the oligo: FRET efficiency falls off with the sixth power of distance. In qPCR 5′-nuclease probes, the dye is at one end of a ~20–30 nt oligo and the quencher at the other end, so they are within ~6–10 nm when the probe is intact (sufficient for quenching with high-efficiency quenchers). Some designs use an internal quencher (placed on a T base in the middle of the oligo) to bring it closer to the fluorophore and improve quenching. This is often done with Dabcyl or BHQ-1 on a T, for instance. The exact placement can affect quenching efficiency and even probe binding, so empirical testing or literature precedent guides these choices.
Quencher Solubility and Chemistry: Like dyes, quenchers can be hydrophobic or hydrophilic. Many dark quenchers are hydrophobic aromatic systems. However, newer ones like BHQ and Dyomics DYQ are formulated to be more polar (some BHQs have a small peg-like linker inherently). A poorly soluble quencher can reduce probe performance (e.g. causing the probe to self-quench or stick to plastic). Thus, modern quenchers are usually chosen for good aqueous behavior. This is one reason Dabcyl has fallen out of favor for solution assays – it is relatively hydrophobic and less efficient; quenchers like BHQ-1 or ATTO 540Q are more effective and easier to work with in water.
In summary, the best practice is to use a quencher specifically matched to your fluorophore’s spectral output and known to be efficient in that role. Many published probe designs and supplier recommendations can be consulted for this. Table 2’s ranges can serve as a general rule-of-thumb for matching. Avoid using a quencher in a spectral region where it absorbs weakly, or you will observe higher background fluorescence.
Application-Specific Considerations
With the general criteria established, we now consider how the priorities might shift for specific applications: qPCR probes, FRET assays, and general oligonucleotide labeling for imaging or detection. Each application imposes particular demands that can influence dye/quencher selection.
qPCR Probes (Real-Time PCR)
In probe-based qPCR (e.g. TaqMan®-type 5′ nuclease assays or molecular beacon probes), a fluorescent reporter and quencher are placed on the oligonucleotide such that the intact probe is dark, and fluorescence is released upon probe cleavage or conformational change. Key considerations for qPCR labels:
Thermostability: As mentioned, probes undergo repeated thermal cycling (typically 40+ cycles of 95 °C and ~60 °C). Dyes and quenchers must not degrade or detach over these cycles. This is crucial for quantitative accuracy between early and late cycles. Use dyes known to withstand PCR conditions (most standard ones do, but newer highly thermostable dyes like ATTO series can provide extra confidence). Quenchers must also be thermostable – BHQ and others were developed for PCR and are stable through many cycles. Avoid labels that are known to be temperature-sensitive. It’s also wise to ensure the oligo-dye linkage is stable (e.g. TAMRA phosphoramidites form a stable bond; some older fluorescein labels could hydrolyze from an oligo if not attached through a stable linkage).
Brightness vs. Instrument Detection: qPCR machines have specific optical channels (often similar to filter cubes in fluorescence plate readers). Common channels are FAM (~520 nm emission), VIC/HEX (~550 nm), TAMRA/ROX (~580–610 nm), and Cy5 (~670 nm) – this allows multiplexing up to four targets. Choose dyes that match the instrument’s available channels. For instance, many instruments use FAM as the high-sensitivity channel (since FAM is very bright); if sensitivity is paramount for a target, put it in the FAM channel. For multiplex, ensure each dye is supported by the machine (some qPCR instruments have fixed filter sets). Also, the dye’s brightness matters because qPCR detects fluorescence in real-time and differences of even 1–2 cycles (which correspond to ~2× differences in target quantity) must be discernible. A brighter dye yields a higher signal-to-noise, enabling detection of small amplification differences. For this reason, FAM is often the go-to for the lowest-abundance target in a multiplex, due to its high brightness. If using a less bright dye (say Cy5 with QY ~0.3), ensure its target is abundant enough or the instrument’s red channel is sensitive enough. New dyes like ATTO 647N can replace Cy5 to give a brighter far-red signal, which can be advantageous in qPCR multiplexing where Cy5’s signal sometimes trails others.
Quencher Choice: qPCR probes almost exclusively use dark quenchers to keep background fluorescence minimal. BHQ-1, BHQ-2, etc., are common. Ensure the quencher matches the dye (e.g. do not quench FAM with BHQ-2; BHQ-1 is better). Many commercial probes use BHQ or a similar proprietary quencher (like Eurogentec’s Eclipse or IDT’s Iowa Black®). From a design perspective, the efficiency of quenching is critical: poor quenching leads to a high baseline fluorescence (ΔRn) which reduces the dynamic range of detection. Ideally, >95% of the reporter signal is quenched in the intact probe. Internal quenchers can help with this (e.g. a ZEN™ double-quenched probe has a second quencher halfway along the oligo to more completely suppress fluorescence). Double-quenched designs are increasingly popular as they lower background fluorescence significantly, allowing earlier cycle threshold determination. If designing your own probes, consider dual quenchers if maximum sensitivity is required.
Oligo Length and Dye Placement: qPCR probe length is typically 20–30 nucleotides. Generally, the reporter is at the 5′ end so that the polymerase 5′→3′ exonuclease will encounter it first and cleave it off during extension, separating it from the quencher. The quencher is often at the 3′ end (with a 3′-end block to prevent extension). This configuration works well for TaqMan probes. Molecular beacons, by contrast, are hairpin probes with the dye and quencher brought together in the stem; they require a quencher that efficiently works at very short range (Dabcyl was historically used for beacons, paired with fluorescein). For beacons, the quencher must be efficient in close proximity (contact quenching can play a role). BHQ dyes also work in beacons and provide broader spectral options than Dabcyl. In any case, ensure the probe configuration (linear vs. hairpin) is compatible with the quenching mechanism of the chosen pair.
Multiplex Considerations: If running multiplex qPCR, choose dye/quencher sets that minimize cross-talk. This includes avoiding FRET between different probes. For example, if one probe’s dye can act as a FRET donor to another probe’s dye (because their spectra overlap and probes might be in proximity in solution), it could cause inter-probe quenching or emission bleed. Typically this is not a big concern in qPCR because probes diffuse freely (low chance of consistent FRET interaction) and because each probe’s fluorescence is monitored in separate channels. Still, spectral bleed-through is the main issue: a strong signal in one channel might appear as a weak signal in another if the spectra overlap. The solution is to use the instrument’s multicolor calibration or compensation if available, or pick dyes with enough separation and proper filters. For instance, FAM and HEX have some overlap; most instruments can computationally subtract HEX bleed in the FAM channel. Using large Stokes shift dyes (if the instrument allows excitation that’s distinct) could be another strategy, but qPCR machines rarely have that flexibility (they use broad-spectrum lamps or a few fixed LEDs/lasers). So, stick to well-established dye sets for multiplex qPCR for predictable results.
In summary, qPCR probes benefit from bright, photostable, thermostable dyes and efficient dark quenchers. Proven combinations (FAM-BHQ1, HEX-BHQ1, ROX-BHQ2, Cy5-BHQ3, etc.) are safe choices. Newer combinations (e.g. ATTO 550 with BHQ-1, ATTO 647N with BHQ-3) can offer performance boosts in brightness and stability. Always validate multiplex assays individually first, and verify that amplification curves and baseline fluorescence behave as expected (flat baseline, exponential increase in correct channel only).
FRET Assays (Donor–Acceptor Pairs)
FRET (Förster Resonance Energy Transfer) experiments involve a donor fluorophore and an acceptor (which may be a fluorescent dye or a dark quencher) in proximity. In oligonucleotide contexts, FRET is used in assays like dual-probe hybridization assays (e.g. adjacent probes on a target DNA, one with donor, one with acceptor), in certain SNP genotyping assays, or for studying interactions (e.g. DNA/RNA folding, protein-DNA interactions by attaching donor and acceptor on the same strand or two strands). Selecting dyes for FRET revolves around some specific criteria:
Spectral Overlap (Förster Criterion): The donor’s emission spectrum must overlap significantly with the acceptor’s absorption spectrum. The degree of overlap directly influences the Förster distance (R₀) – the distance at which energy transfer efficiency is 50%. A larger overlap integral yields a larger R₀. For instance, a classic FRET pair is fluorescein (donor) and tetramethylrhodamine (acceptor); fluorescein’s emission around 520 nm overlaps well with TAMRA’s absorption (peak ~555 nm), giving R₀ on the order of ~5–6 nm for that pair. If the overlap is poor, R₀ will be small and FRET will only occur at very short distances. Manufacturers like ATTO-TEC provide R₀ tables for various dye combinations. For example, ATTO has calculated R₀ for all combinations of their dyes under standard conditions (κ^2 = 2/3, random orientation). These tables are a great resource to pick an optimal FRET pair – one would choose the pair with the highest R₀ (longest distance) that suits their spectral needs. Typically, you want R₀ at least on the order of the expected donor–acceptor distance in your system to see efficient transfer.
Donor Quantum Yield: FRET efficiency is proportional to the donor’s quantum yield (among other factors). A high QY donor will transfer energy more effectively because it has more excitation energy to potentially give away. Therefore, a bright donor is beneficial. However, note that once attached to an acceptor (especially in close proximity), the donor’s apparent fluorescence will diminish due to transfer – this is expected. One often measures FRET by either acceptor emission increase or donor emission quenching.
Acceptor Extinction Coefficient: A high absorption by the acceptor at the donor emission wavelength is critical (which is the flip side of spectral overlap). If using a fluorescent acceptor, its quantum yield also matters for its emission brightness.
Distance and Orientation: FRET efficiency drops with the sixth power of distance and depends on the relative orientation of the fluorophores’ transition dipoles (κ^2 factor). In oligonucleotides, the dyes are often linked via flexible tethers, and they can rotate somewhat freely, so an assumption of random orientation (κ^2 = 2/3) is usually made. Still, if dyes intercalate or stack with bases, orientation can become non-random. For example, certain cyanines (Cy3, Cy5) can stack onto double-stranded DNA ends, potentially affecting κ^2 and thus FRET. Rigid linkers or positioning in a duplex vs. single-strand can alter the effective κ^2. For practical selection, one mostly focuses on spectral overlap and distance, but be aware that measured FRET efficiencies may differ from ideal calculations due to orientation or environmental effects. If precise distances are to be inferred from FRET, consider dyes known to behave well (e.g. Alexa or ATTO dyes that stay sufficiently flexible) and avoid cases where one dye might stack.
Photostability in FRET: In many FRET assays (e.g. real-time detection with hybridization probes or single-molecule FRET), photostability is important. If the donor photobleaches, you lose both donor and FRET signal. If the acceptor photobleaches (and donor remains), FRET will cease and donor fluorescence will jump (often an observed indicator in single-molecule studies). Using photostable dyes prolongs the observation window. ATTO dyes have been successfully used in single-molecule FRET due to their high photostability. On the other hand, classic Cy5 is less photostable, which can be a limitation in extensive imaging. So for any extended FRET monitoring, lean towards the more photostable fluorophores for both donor and acceptor.
Dual vs. Single-Labeled Probes: In some cases like qPCR with adjacent hybridization probes (e.g. LightCycler® probes), you have two separate oligos: one with a donor at 3′ end, another with an acceptor at 5′ end. When they bind adjacent on a target, FRET occurs. For such systems, it’s typical to use a high-QY donor (e.g. fluorescein or an analog) and a reasonably bright acceptor (often a red dye like LC-Red640 or Cy5). If the acceptor is fluorescent, the qPCR machine reads its emission (FRET-sensitized emission) as a function of target binding. If the acceptor is nonfluorescent (dark quencher), then one monitors the donor’s fluorescence quenching instead (less common in qPCR, but used in some probes). The design choice dictates whether you need a fluorescent acceptor or not. Fluorescent acceptors allow ratiometric measurements (you can potentially measure both donor and acceptor channels for more info), but complicate the optics.
Separation of Emission: If using a fluorescent acceptor, ensure the acceptor’s emission can be distinguished from the donor’s. Typically, you choose a FRET pair that is fairly far apart in emission color – e.g. a green donor with a red acceptor. There will always be some bleed-through of donor emission into the acceptor channel if the spectra are not fully distinct. Correct this by proper filter choice or post-processing (spectral unmixing). Some newer FRET pairs try to use very large Stokes shift donors to get even more separation. For example, a coumarin donor excited in UV with emission in blue, transferring to a green/yellow acceptor, can give a large separation. The downside is UV excitation and often lower donor brightness. Most common FRET pairs are roughly 100 nm apart in emission.
Calibration and Controls: In a FRET experiment’s context, selecting the dyes is one part; equally important is calibrating the system (for instance, measuring donor bleed-through and acceptor direct excitation to subtract those from the FRET signal). When picking dyes, try to minimize those unwanted signals: ideally the acceptor has minimal direct excitation by the donor’s excitation source. For instance, if using a single excitation at 480 nm for CFP/YFP, YFP (acceptor) will also absorb some at 480 nm and fluoresce – that’s direct excitation leakage. If that is significant, you have to correct for it. By selecting an acceptor that doesn’t appreciably absorb the donor excitation (which may conflict with needing overlap of donor emission – it’s a balancing act), you can reduce that artifact. Many FRET studies accept some direct excitation and correct mathematically.
Reference Data: It can be useful to consult literature or provided data for known FRET efficiencies. ATTO-TEC’s downloadable R₀ spreadsheet can guide your choices – for example, it might tell you ATTO 488 donor with ATTO 565 acceptor has R₀ = X nm, whereas ATTO 488 with ATTO 590 has R₀ = Y nm. You’d pick the higher R₀ for better sensitivity, all else equal, unless there’s another reason (like instrument detection) to choose the other.
In summary, select a donor–acceptor pair with a large R₀, high individual brightness, and appropriate emissions for your detection capabilities. A classic set for oligo FRET is fluorescein–Cy5 (green to red) which has an R₀ ~50–60 Å in buffer; other popular pairs: CFP–YFP for protein FRET (cyan to yellow, ~50 Å), or newer pairs like Alexa 488–Alexa 594, ATTO 550–ATTO 647N, etc., which often improve on the classics in brightness and stability. Ensure your oligo design places them at a suitable distance (e.g. within 1–5 nucleotides for intramolecular FRET, or on adjacent binding probes for intermolecular FRET). With careful pair selection and proper controls, FRET-labeled oligos can provide dynamic information on molecular interactions and distances with high sensitivity.
General Oligonucleotide Labeling (Imaging, FISH, and Other Uses)
Beyond qPCR and specialized FRET assays, fluorescent oligonucleotides are widely used as probes and tags in techniques like fluorescence in situ hybridization (FISH), microarray analysis, flow cytometry, and general microscopy imaging of nucleic acids. These applications have their own nuances for dye selection:
Fluorescence In Situ Hybridization (FISH): FISH involves hybridizing fluorescently labeled DNA probes to targets in fixed cells or tissues (e.g. chromosome painting, mRNA detection). Key here is brightness and photostability, because often targets may be present in low copy, and samples are viewed under a microscope. Typically, multiple different probes are used in one sample (different colors for different targets), so multiplexing without crosstalk is important. Far-red dyes are especially valued in FISH, because tissue auto-fluorescence is strong in blue/green but much lower in far-red. Using, say, Cy5 or ATTO 647N labeled probes can yield a high contrast signal with low background from the specimen. Additionally, far-red light is less damaging to the sample (important for preserving morphology). Common FISH dyes include FITC, Texas Red, Cy3, Cy5, but these are being supplanted by more stable dyes like Alexa Fluor® series or ATTO series to withstand the long imaging sessions and archival storage. Ozone stability can be an unsung factor in FISH as well – slides might be stored or mailed, so using an ozone-resistant dye (ATTO 655/647N rather than Cy5) can preserve signal. If multiple rounds of imaging or bright-field/fluorescence combinations are done, photostability is crucial to avoid signal fading during acquisition. For instance, using ATTO 488 instead of FITC can maintain fluorescence longer through image z-stacks because ATTO 488 is far more photostable than FITC (which can bleach significantly in seconds). In summary for FISH: use bright, stable dyes (even if more expensive), and pick wavelengths that minimize tissue autofluorescence. A typical 3-color FISH might use ATTO 488 (green), Rhodamine or Alexa 594 (red), and ATTO 647N or Alexa 647 (far-red) – each well separated and very bright.
Microarrays and High-Throughput Hybridization Assays: DNA microarrays traditionally used Cy3 and Cy5 as the two fluorophores for two-channel expression analysis. As noted, Cy5 on microarray slides suffered from ozone degradation, leading to artifactual differences. Replacing Cy5 with a more stable dye dramatically improves data consistency. Companies introduced Cy5 analogs (Alexa 647, ATTO 647N, HyPer5, etc.) to address this. For any surface-bound oligo application (microarrays, spatial transcriptomics slides, etc.), consider ozone stability and surface binding effects. Dyes at surfaces are more exposed to air (ozone) and also cannot be regenerated (photobleached spots cannot be recovered). ATTO 647N’s 100-fold ozone stability advantage over Cy5 is a compelling reason to choose it for microarray labeling. Likewise, ATTO 655 or DyLight 650 have been used as drop-in Cy5 replacements with better stability. Cy3 is less sensitive to ozone than Cy5 but still can bleach; alternatives like Alexa 555 or ATTO 565 can be more photostable. In one study, Cy5 and ATTO 647N were compared and both produced strong signals, but Cy5 signals dropped significantly with even brief ozone exposure. Bottom line: if doing microarrays, strongly consider using optimized dyes (many array kit providers now supply Alexa or ATTO dyes in place of Cy dyes). Also, work in a low-ozone environment or use anti-ozone slides if possible, but dye choice is the most straightforward fix.
Flow Cytometry with Oligo Probes: Sometimes fluorescent oligonucleotides are used in flow cytometry (e.g. as barcoded probes attached to beads, or aptamers with a fluorescent tag binding to cells). The principles here align with those for fluorescent antibodies: brightness and appropriate spectral matching to the cytometer’s lasers and filters. If you attach an oligo to a bead or cell, you generally can load many fluorophores per target (multiple oligos or multiple dyes on one oligo), so brightness can be amplified. But using an inherently bright dye helps maximize signal. Also, consider if the oligo-dye will see any extreme conditions (some flow assays might involve 37 °C incubations, etc., which most dyes handle, or pH changes if going into endosomes). Ensure the dye is stable in whatever buffer is used (some dyes precipitate if the buffer is not good – e.g. certain cyanines in low salt can aggregate; including some salt or solvent can help). Flow cytometry is forgiving in that signals are averaged over many particles, but you want robust signals from each. Thus, again, favor high-extinction/high-QY dyes (e.g. ATTO 550 instead of Rhodamine 6G, or Alexa 647/ATTO 647N instead of Cy5 for far-red). These choices can give more intense fluorescence per molecule.
General Imaging (Fluorescent in vitro assays, gel imaging, etc.): Fluorescent oligos are used as primers in some assays or as markers on gels (e.g. to visualize size ladders). If an oligo will be subjected to enzymatic reactions (like a fluorescent primer in PCR for fragment analysis), thermal stability again matters (choose a dye stable to PCR). For gel-based detection (e.g. a fluorescein-labeled oligo in a gel shift assay), brightness and the ability to be detected by the imager (laser lines available) matter. Often imagers have strong lasers (488, 532, 635 nm, etc.) and sensitive cameras, so most dyes will work; just ensure you pick one matching the imager filters. One must also consider if the dye might affect the biological activity of the oligo: for example, a fluorescent oligo meant to bind a protein might have altered affinity due to the bulky dye. In such cases, smaller dyes or positioning the dye at a terminus (away from the binding sequence) is advised. Some applications use multiple dyes on one oligo (to boost brightness or create specific signatures). If attaching multiple dyes, be aware of self-quenching: identical fluorophores in close proximity can quench each other (through homo-FRET or contact). This is sometimes intentional (e.g. in quenched FRET probes that light up when dyes separate), but if your goal is simply a brighter probe, spreading the dyes out or using a spacer can mitigate quenching. Alternatively, using a dye with a large Stokes shift can reduce quenching among identical dyes by lessening re-absorption of one dye’s emission by another in the vicinity.
Multi-Color Combinatorial Probes: In some advanced applications like spectral barcoding, oligos are labeled with combinations of dyes to create unique spectral “codes.” In these, it’s crucial that each dye’s contribution is well-characterized and that they do not quench one another. Typically, dyes that are spectrally separated are attached, or if multiple of the same dye are attached, they ensure no quenching by spacing. The selection here gets very complex, but the general guidance holds: choose dyes that collectively can be distinguished and that remain stable on the oligo.
Storage and Handling: Once you have your fluorescent oligonucleotide, how you store it can affect the dye. Many fluorophores (especially cyanines) are light-sensitive – always store labeled oligos in the dark (wrap tubes in foil). Some are a bit unstable in basic solutions over long term (e.g. fluorescein can get slowly hydrolyzed at high pH). It’s common to store probes in TE buffer (pH ~8) at –20 °C; most dyes will be fine for months/years at this condition. Avoid repeated freeze-thaw; aliquot if possible. If you notice a probe losing signal over time, consider aliquoting into a low-adsorption tube and possibly adding a stabilizer (some people add 1 mM sodium azide to prevent microbial growth and 50% glycerol to avoid freezing altogether). For critical probes, manufacturing fresh or buying from reputable suppliers who ensure proper handling is worthwhile.
For general oligonucleotide labeling tasks, the guiding principle is to choose the brightest, most stable dye that fits your detection setup, and to consider any special environmental challenges (pH, ozone, temperature) the probe will face. Quenchers, if used, should be matched and efficient. The cost may be a factor, but often the dye is not the cost driver in an experiment – poor results are far more costly. Thus, leveraging the advances made by dye chemists (ATTO, Dyomics, and others) can significantly boost the performance of oligo-based assays. The tables and criteria above should serve as a roadmap to making an informed selection. By carefully considering brightness, photostability, spectral overlap, and compatibility, researchers can ensure their fluorescently labeled oligonucleotides deliver strong, reliable signals in whatever application they pursue.
References:
ATTO-TEC GmbH – How to Choose a Label: Key factors for optimal fluorescent labeling (excitation source, absorption, quantum yield, etc.)atto-tec.comatto-tec.com.
ATTO-TEC GmbH – Properties of Fluorescent Labels: Discussion of pH dependence, photostability (FITC vs ATTO dyes), and ozone stability in dyesatto-tec.comatto-tec.com.
ATTO-TEC GmbH – Fluorescence Quenchers: Description of ATTO’s dark quencher series (high extinction coefficients and photostability)atto-tec.com.
ATTO-TEC GmbH – ATTO 647N Product Data: Optical characteristics (λ_abs 646 nm, ε=1.5×10^5, Φ=65%) and noted high thermal, photo, and ozone stability; pH-independence of ATTO 647Natto-tec.comatto-tec.com.
Addgene Blog – Guide to Selecting Fluorescent Dyes: Overview of brightness (ε and Φ) and Stokes shift concepts; example of brightness difference (DAPI vs Alexa 488)blog.addgene.org and definition of Stokes shiftblog.addgene.org.
Glen Research Technical Note – Fluorescence Data: Tabulated extinction coefficients and quantum yields for common dyes and quenchers (e.g. FAM, HEX, TAMRA, Cy3, Cy5, Dabcyl, BHQ series)glenresearch.comglenresearch.com.
Dyomics GmbH – Product Catalogue (8th Ed.) Announcement: Introduction of PEGylated dyes for reduced non-specific binding and new MegaStokes dyesdyomics.com.
Sednev et al. (2015) – Large Stokes Shift Dyes Review: Noted value of large Stokes shift fluorophores for multiplexing (reduced cross-talk)scispace.com and the relative rarity of bright, photostable large-Stokes dyes (often coumarin-based; e.g. Dyomics ‘MegaStokes’)scispace.com.
Satterfield et al. – Photobleaching in Microarrays: Explanation that photobleaching is caused by light and ozone and varies by fluorophoremdpi.com, underlining the need for stable dyes in microarray experiments.
Alexa Fluor® Dyes – Succinimidyl Esters (NHS Esters). (2012, October 16). Technical Data Sheet, MP10168. Life Technologies.
(For photostability, excitation/emission characteristics, and NHS conjugation chemistry)
[Document: AlexaFluor Specification sheet.pdf]AZDye 594 NHS Ester – Product Information Sheet. (n.d.). Fluoroprobes LLC.
(Spectral properties and comparison to Alexa Fluor® 594, CF®594, DyLight®594)
[Document: AZDye594_identisch_zu_Alexa594.pdf]Atto-Tec NHS Esters – Fluorescent Labels for Biomolecule Conjugation. (n.d.). ATTO-TEC GmbH.
(For dye structure, reactivity, pH dependence, solubility, and application compatibility)
[Document: Atto-Tec NHS.pdf]ATTO-TEC Fluorescent Labels – 2023–2024 Product Catalogue. (2023). ATTO-TEC GmbH.
(Comprehensive dye selection guide: emission spectra, conjugation chemistries, application notes)
[Document: Atto-Tec_2023_2024.pdf]Dyomics Fluorescent Dyes for Bioanalytical and Hightech Applications – 8th Edition. (2017). Dyomics GmbH.
(Spectral characteristics of DY-labels, including MegaStokes dyes and pH/solvent effects)
[Document: Dyomics_2017.pdf]Martin, M. M., & Lindqvist, L. (1975). The pH dependence of fluorescein fluorescence. Journal of Luminescence, 10(6), 381–390.
https://doi.org/10.1016/0022-2313(75)90072-7
(For foundational data on pH-dependent fluorescence of fluorescein)
[Document: The pH dependence of fluorescein fluorescence - martin1975.pdf]Fluorophores and Cyanines: Fluorescent Properties and Application Guidelines. (n.d.). Fluoroprobes LLC.
(For cyanine dye comparison, photostability, FRET compatibility, and NHS chemistry overview)
[Document: FPCY_Fluorphores_Cyanines.pdf]






Great compilation of information!