Synthetic tRNAs in Protein Engineering: Redefining Protein Structure and Function
Scientific Digital Marketing, Synthetic Biology, Nucleic Acid Therapeutics and Antibody Engineering, Biotech Writer
The ability to design and synthesize proteins with unique properties has revolutionized biotechnology, enabling applications that range from therapeutic development to advanced material science. Central to this innovation is the field of synthetic biology, which employs engineered genetic circuits, artificial nucleotides, and synthetic transfer RNAs (tRNAs) to expand the natural genetic code. Synthetic tRNAs are at the forefront of this technology, acting as precise delivery vehicles for non standard amino acids (nsAAs) into specific sites of a protein. This strategy is transformative because it allows researchers to move beyond the constraints of the 20 canonical amino acids encoded by the standard genetic code. By strategically reassigning codons and using orthogonal tRNA synthetase pairs, synthetic tRNAs enable the precise incorporation of novel amino acids, paving the way for proteins with tailored chemical and physical properties. This article will explore how these innovations are implemented and the principles that guide their design.
In the standard genetic code, 64 codons encode just 20 canonical amino acids, leaving significant redundancy and unused coding potential. Synthetic biology exploits this redundancy to assign new meanings to codons, incorporating nsAAs with properties like fluorescence, cross linking reactivity, or unique electronic characteristics. This process requires precise molecular engineering of synthetic tRNAs, along with complementary aminoacyl tRNA synthetases (aaRSs) that can charge the tRNAs with nsAAs. The article discusses in depth how tRNA codon reassignment and orthogonal tRNA synthetase pairs function to ensure specificity and accuracy in translation. The ability to reassign codons, engineer orthogonal systems, and incorporate nsAAs has created exciting possibilities in protein engineering, from therapeutic proteins to de novo protein structures.
The use of synthetic tRNAs has led to groundbreaking advances in protein design, enabling the creation of enzymes with light controlled activity, antibodies with enhanced stability and drug binding capabilities, and proteins that self assemble into nanostructures. Beyond single site modifications, synthetic tRNAs have been used to design entirely new protein architectures through the incorporation of expanded genetic codes into cells or cell free systems. This article will delve into the chemical and biological methods for charging tRNAs, the tools for integrating synthetic tRNAs into biological systems, and the strategies for overcoming challenges like codon competition and system inefficiencies. These approaches have unlocked the ability to control protein function and structure with unprecedented precision.
Despite the remarkable progress in using synthetic tRNAs, challenges remain in their efficient application. Competition with endogenous translation machinery, host toxicity, and limitations in translation fidelity are significant hurdles. However, ongoing innovations such as orthogonal ribosomes, quadruplet codons, and machine learning driven design are rapidly addressing these barriers. As discussed in this article, the development of high fidelity systems, advanced genetic circuits, and expanded codon usage are enhancing the efficiency and versatility of synthetic tRNA systems. With these advancements, synthetic tRNAs are poised to become an indispensable tool in creating proteins with novel functionalities, redefining the boundaries of synthetic biology and protein engineering.
tRNA Structure and Function
Transfer RNAs (tRNAs) are small RNA molecules that serve as essential adaptors in the process of translation, bridging the genetic information encoded in messenger RNA (mRNA) with the corresponding amino acids required for protein synthesis. Their highly conserved structure and precise functionality make them indispensable in ensuring the accuracy and efficiency of translation.
Structure of tRNA
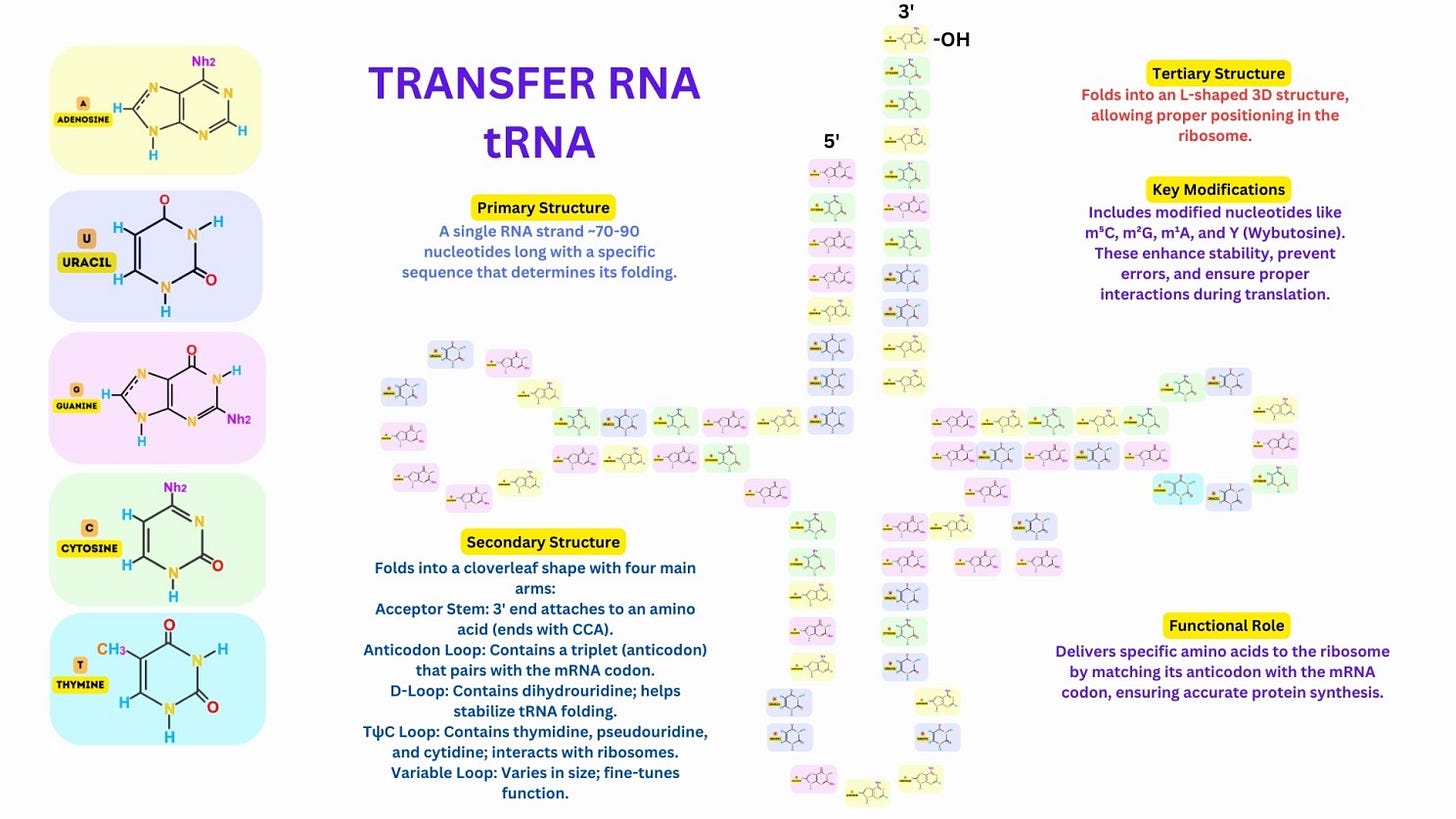
Primary Structure: A tRNA molecule consists of a single RNA strand, typically 70–90 nucleotides long, with a specific sequence that determines its folding and function.
Secondary Structure:
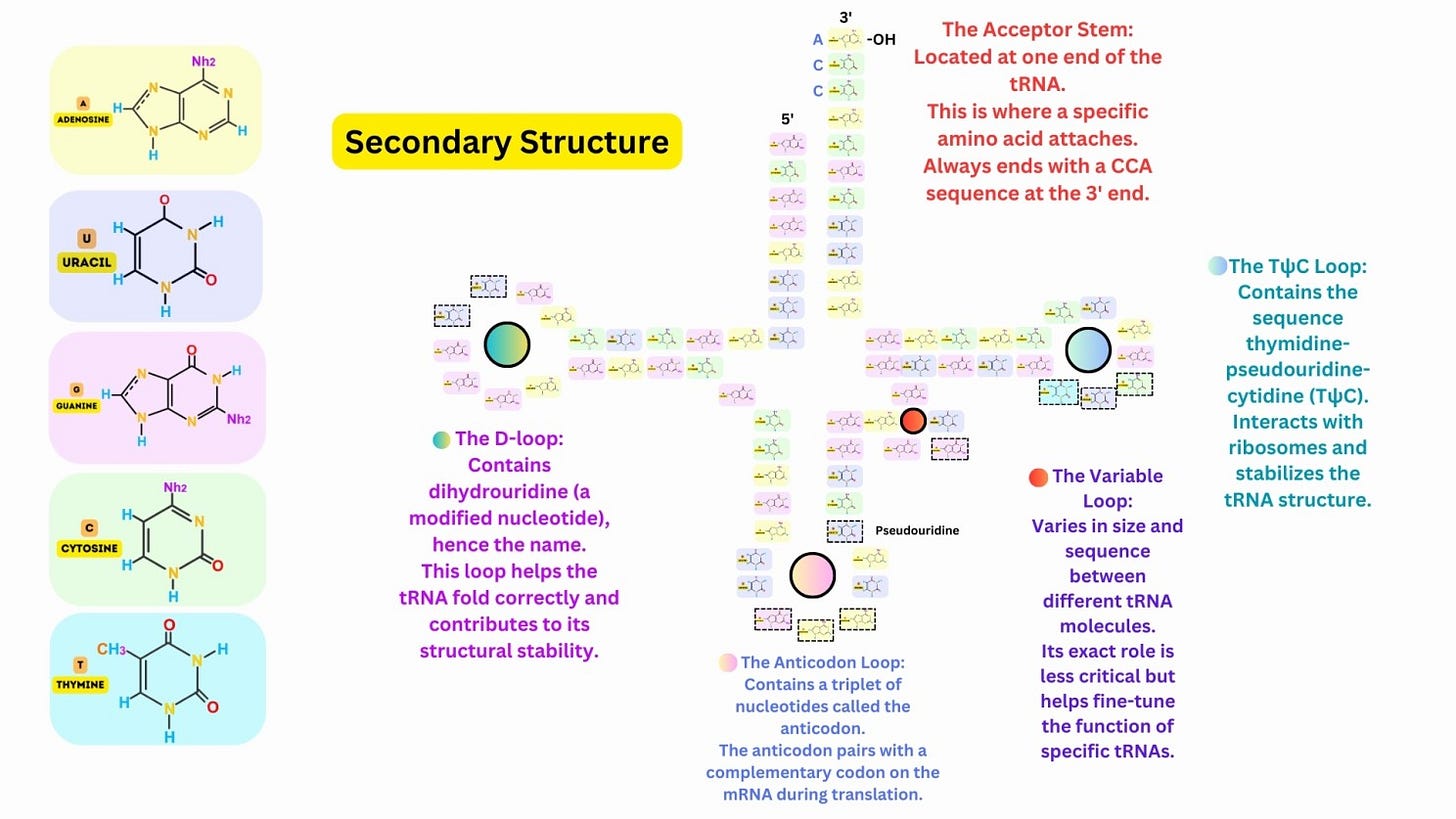
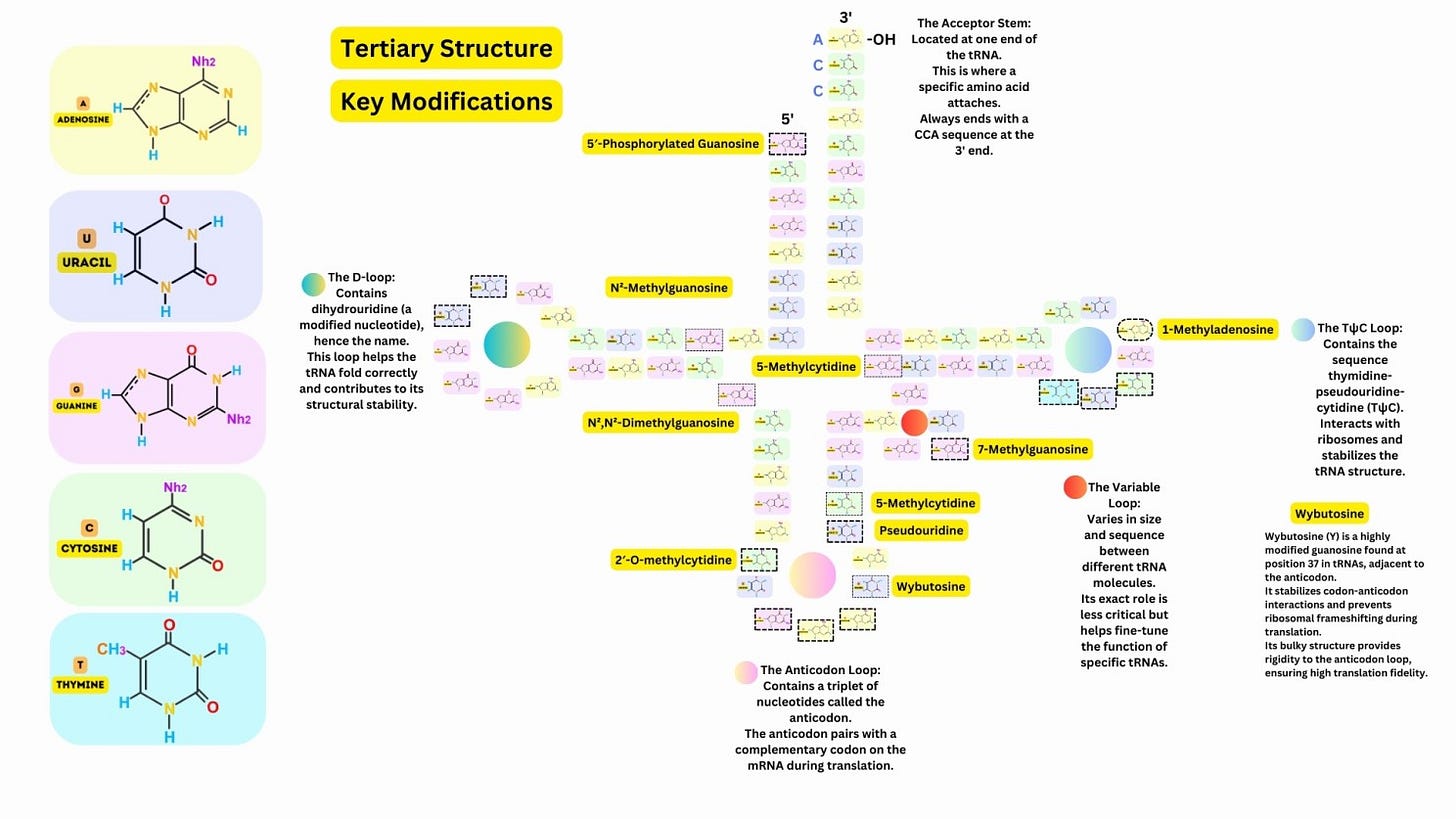
The tRNA folds into a characteristic cloverleaf shape, with four main regions: Acceptor Stem: At the 3′ end, where the amino acid is attached. Anticodon Loop: Contains a three-nucleotide anticodon that pairs with the complementary mRNA codon. D-Loop: Contains dihydrouridine, which stabilizes the folding of the tRNA. TψC Loop: Contains pseudouridine and stabilizes interactions with ribosomes. A variable loop is present in some tRNAs, contributing to functional diversity.
Tertiary Structure:
The secondary structure folds further into an L-shaped three-dimensional structure, where one arm carries the anticodon and the other carries the amino acid, ensuring precise interaction with the ribosome.
Function of tRNA
tRNAs play a critical role in translation by:
Carrying Amino Acids: Each tRNA is charged with a specific amino acid at its 3′ end by its corresponding aminoacyl-tRNA synthetase (aaRS), ensuring accurate pairing between the anticodon and the mRNA codon.
Decoding mRNA Codons: The anticodon loop recognizes and binds to complementary mRNA codons, aligning the attached amino acid for incorporation into the growing polypeptide chain.
Facilitating Ribosome Functionality: The L-shaped structure positions the amino acid within the ribosome's catalytic core, enabling peptide bond formation during protein synthesis.
Linking tRNA Structure and Function to Synthetic Modifications
The fundamental structure and function of tRNAs make them ideal targets for synthetic modifications, enabling novel capabilities in protein engineering. By altering specific regions of tRNA or its interactions, synthetic biology leverages the natural efficiency and precision of tRNA functionality while expanding its potential beyond canonical protein synthesis.
How Synthetic Modifications to tRNA Enable Novel Protein Engineering
Codon Reassignment: Modifying the anticodon loop allows tRNAs to recognize codons reassigned to encode non-standard amino acids (nsAAs), such as stop codons (e.g., UAG) or rare codons.
Incorporation of nsAAs: Engineering tRNAs to pair with orthogonal aminoacyl-tRNA synthetases (aaRSs) enables the addition of amino acids with unique chemical, fluorescent, or catalytic properties into proteins.
Orthogonal Systems: Altering the acceptor stem and D-loop prevents synthetic tRNAs from interacting with host aaRSs, ensuring that synthetic systems function independently of the host's natural translation machinery.
Expanded Codon Usage: Engineering tRNAs to decode quadruplet or artificial codons dramatically increases the number of amino acids that can be encoded, enabling de novo protein design.
Chemical Labeling: Synthetic tRNAs can be chemically charged with amino acids or functional groups that natural aaRSs cannot handle, such as photo-reactive groups or bioconjugation tags.
Protein Stabilization and Functionality: Modifications to tRNAs enable precise placement of nsAAs in proteins, allowing the creation of proteins with enhanced stability, catalytic properties, or tailored allosteric behavior.
By integrating synthetic tRNAs into systems that exploit these modified functionalities, researchers can:
Design enzymes with novel catalytic activities that respond to external stimuli, such as light or chemical signals.
Create therapeutic proteins with improved pharmacokinetics, such as antibodies with enhanced stability and drug-conjugation sites.
Develop de novo protein architectures for biomaterials, such as self-assembling nanostructures and hydrogels.
Expand the genetic code to include dozens of new amino acids, facilitating the study of protein structure and function in unprecedented detail.
This integration of synthetic tRNA modifications into protein engineering highlights the transformative potential of these technologies, as discussed throughout the article. It is the convergence of natural precision and synthetic innovation that drives the field forward, unlocking possibilities previously unimaginable in biological systems.
Key Principles of Synthetic tRNA Engineering
tRNA Codon Redundancy and Reassignment
The design of synthetic tRNAs builds on the inherent flexibility and redundancy of the genetic code, allowing novel amino acids to be incorporated into proteins with precision.
tRNA Codon Redundancy
The genetic code has 64 codons, yet only 20 standard amino acids are encoded. This creates redundancy, where multiple codons encode the same amino acid (referred to as synonymous codons). For instance:
Glycine: Encoded by GGG, GGC, GGA, GGU.
Leucine: Encoded by UUA, UUG, CUU, CUC, CUA, CUG.
This redundancy provides "spare" codons that can be reassigned without disrupting the coding of essential proteins.
Codon Reassignment
Reassignment involves redirecting the interpretation of specific codons to incorporate non standard amino acids (nsAAs) instead of their native assignments.
Stop Codon Reassignment: Stop codons (UAG, UAA, UGA) are particularly useful for reassignment because they do not naturally encode amino acids. The amber stop codon (UAG) is a frequent target for reassignment because it is less commonly used in many organisms, minimizing the disruption to normal translation.
Rare Codon Reassignment:
Rare codons that are seldom used in the host's genome can be reassigned. For example, a rare leucine codon (CUG) could be reassigned to incorporate an nsAA, provided the endogenous tRNAs recognizing CUG are removed or silenced.
Quadruplet Codon Systems:
Engineering ribosomes to read quadruplet codons (e.g., AGGA or CUAG) instead of standard triplets dramatically expands the codon space. This allows entirely new codons to be assigned to nsAAs without competing with existing ones.
Mechanisms of Reassignment
Codon reassignment requires three critical components:
Synthetic tRNA: A synthetic tRNA is designed with an anticodon that matches the codon to be reassigned (e.g., UAG for amber stop codon). This tRNA is introduced into the system, typically via a plasmid or integrated into the genome.
Orthogonal Aminoacyl tRNA Synthetase (aaRS):
An engineered aaRS specifically recognizes the synthetic tRNA and its assigned nsAA.
Orthogonality ensures that the synthetic tRNA and nsAA do not interfere with the host's natural translation machinery.
Host System Engineering:
Endogenous tRNAs or release factors (e.g., RF1 for UAG stop codons) that recognize the reassigned codon are removed or suppressed. For example, in E. coli, the deletion of RF1 allows the amber codon (UAG) to function exclusively as a reassigned codon.
Example: Amber Stop Codon Reassignment
Native Function: The UAG codon signals translation termination in standard genetic codes.
Reassigned Function: In engineered systems, a synthetic tRNA with an anticodon complementary to UAG (CUA) can be charged with an nsAA and inserted into proteins.
Steps in Amber Codon Reassignment:
Design a synthetic tRNA with the anticodon sequence CUA, complementary to the UAG codon.
Engineer an orthogonal aaRS that charges the tRNA with a specific nsAA.
Introduce the synthetic tRNA/aaRS pair into the host organism (e.g., E. coli).
Remove RF1 or other competing factors to ensure UAG is exclusively recognized by the synthetic tRNA.
Introduce a gene with strategically placed UAG codons to incorporate the nsAA at desired protein sites.
Advantages and Challenges of Codon Reassignment
Advantages
Site Specific Incorporation: Allows precise placement of nsAAs in proteins for unique structural or functional properties.
Expanded Codon Space: Quadruplet codons or reassigned stop codons can encode many more nsAAs than the natural genetic code.
Customizable Protein Functionality: Enables integration of amino acids with novel chemical, fluorescent, or catalytic properties.
Challenges
Codon Competition: Endogenous tRNAs or release factors may compete for the reassigned codon, reducing efficiency.
Host System Toxicity: Engineering the system to suppress natural components (e.g., RF1) can impose a metabolic burden.
Translation Fidelity: Reassignment must avoid introducing errors or frameshifting during translation.
Orthogonal tRNA Synthetase Pairs
To ensure accurate incorporation of nsAAs:
Synthetic biology uses orthogonal tRNA aminoacyl tRNA synthetase (aaRS) pairs, which function independently of the host's natural tRNA synthetase machinery.
These pairs specifically recognize the synthetic tRNA and its associated nsAA, avoiding cross reactivity with endogenous tRNAs.
Key Components of Orthogonal tRNA Synthetase Pairs
Orthogonal tRNA (o tRNA)
Structure: A tRNA engineered or selected to be unrecognized by the host's endogenous aminoacyl tRNA synthetases (aaRS).
Anticodon: Modified to pair with a specific reassigned codon (e.g., amber stop codon UAG or quadruplet codons).
Recognition: Exclusively recognized by its matching orthogonal aaRS.
Orthogonal Aminoacyl tRNA Synthetase (o aaRS)
Function: An enzyme that specifically charges the o tRNA with an nsAA.
Active Site Engineering: Modified to accept nsAAs while maintaining high specificity.
Orthogonality: Does not interact with host tRNAs or amino acids, preventing mischarging.
Mechanism of Action
Recognition: The o aaRS recognizes its cognate o tRNA through unique structural motifs in the tRNA's acceptor stem, D loop, or other elements. Host tRNAs and o tRNAs are mutually exclusive in their interactions with aaRS enzymes.
Charging:
The o aaRS catalyzes the attachment of an nsAA to the 3′ end (CCA) of the o tRNA, forming an aminoacyl tRNA.
Translation:
The otRNA, carrying the nsAA, recognizes its assigned codon on the mRNA and participates in translation at the ribosome. The nsAA is incorporated into the growing polypeptide chain at the site specified by the codon.
Design and Engineering of Orthogonal Systems
Orthogonal tRNA Design
Anticodon Modification: The anticodon is engineered to pair with the desired codon (e.g., UAG for amber stop codon reassignment). Additional modifications may support recognition of quadruplet codons (e.g., AGGA).
Structural Optimization:
Mutations in the tRNA body (e.g., acceptor stem, T loop) prevent recognition by endogenous aaRS while maintaining compatibility with o aaRS.
Orthogonal aaRS Engineering
Active Site Remodeling: The binding pocket is redesigned to accommodate the unique structure of nsAAs. For example, mutations in the active site can increase the binding affinity for bulky side chains, aromatic groups, or reactive functional groups.
Library Screening:
Directed evolution techniques, such as random mutagenesis and high throughput screening, identify aaRS variants that efficiently charge otRNA with specific nsAAs.
Phage display or fluorescence based assays are commonly used for screening.
Elimination of Crosstalk:
Ensuring the engineered aaRS does not charge host tRNAs by refining the aaRS tRNA recognition interface.
Strategies for Orthogonality
Achieving orthogonality between the synthetic system and the host requires:
Host Dependent Engineering: Selecting or evolving tRNA aaRS pairs from organisms evolutionarily distant from the host (e.g., archaeal tRNA aaRS pairs in E. coli). Ensuring minimal sequence overlap with host tRNAs or aaRS.
Artificial Ribosomes:
Orthogonal ribosomes (oRibs) can be engineered to exclusively translate mRNA containing reassigned codons, further isolating synthetic translation from the host system.
Applications of Orthogonal tRNA Synthetase Pairs
Incorporation of Non Standard Amino Acids (nsAAs)
Site specific incorporation of nsAAs with unique properties, such as: Fluorescent labels (e.g., BODIPY conjugated amino acids). Chemical reactivity (e.g., azides, alkynes for click chemistry). Post translational mimicry (e.g., phosphorylated or glycosylated amino acids).
Quadruplet Codon Systems
Pairing o tRNAs with orthogonal ribosomes enables translation of quadruplet codons, vastly increasing the genetic code's capacity to encode novel amino acids.
Synthetic Circuits
otRNA aaRS pairs can be integrated into synthetic genetic circuits for dynamic control of protein function.
Example: Incorporation of light sensitive nsAAs for optogenetic protein activation.
Therapeutic Applications
Site specific conjugation of drugs or imaging agents to therapeutic proteins via nsAAs.
Example: Antibody drug conjugates with precise drug attachment points.
Challenges and Solutions
Challenges
Efficiency: Inefficient charging of of tRNA with nsAAs can limit protein yield. Solution: Use directed evolution to optimize o aaRS activity.
Host Compatibility
Host metabolic burden from overexpression of orthogonal components.
Solution: Optimize expression levels and codon usage.
Crosstalk:
Misrecognition between endogenous and orthogonal systems.
Solution: Rigorous sequence divergence and active site specificity in aaRS.
Example Orthogonal Systems
Methanocaldococcus jannaschii (M. jannaschii) o tRNA/aaRS Pair: Widely used in E. coli for nsAA incorporation. The aaRS tRNA pair is derived from an archaeal organism, ensuring minimal cross reactivity.
Pyrococcus horikoshii Systems:
High thermostability of o aaRS and tRNA, beneficial for certain applications.
Engineered E. coli Systems:
Endogenous aaRS and release factors are knocked out or suppressed to accommodate orthogonal pairs.
Future Directions
Expanding Codon Space: Designing ribosomes that read synthetic codons (e.g., pentuplet codons) paired with o tRNA aaRS systems.
Multiplexed Systems
Using multiple orthogonal pairs to simultaneously incorporate several nsAAs into the same protein.
Machine Learning in Design:
AI driven modeling to predict optimal tRNA aaRS pairs and active site mutations for novel nsAAs.
Chemical and Biological Charging of tRNAs
Synthetic tRNAs are charged with nsAAs using:
Enzymatic Charging: An engineered aaRS catalyzes the attachment of an nsAA to the synthetic tRNA. Example: E. coli tyrosyl tRNA synthetase has been modified to incorporate nsAAs with aromatic side chains.
Chemical Charging
The nsAA is chemically ligated to the synthetic tRNA in vitro, bypassing the need for aaRS enzymes.
The charging of tRNAs, whether natural or synthetic, involves the attachment of an amino acid (standard or non standard) to the tRNA molecule at its 3' terminal adenosine (A76). This process, known as aminoacylation, can occur through two primary mechanisms: biological charging (enzymatic) and chemical charging (non enzymatic). Both methods have specific roles in expanding the genetic code and enabling precise control over protein synthesis.
Biological Charging of tRNAs
Biological charging relies on enzymes called aminoacyl tRNA synthetases (aaRSs) to catalyze the attachment of an amino acid to the tRNA.
Steps in Biological Charging
Activation of the Amino Acid: The aaRS activates the amino acid by reacting it with ATP, forming an aminoacyl adenylate intermediate (amino acid AMP). This intermediate is highly reactive and primes the amino acid for transfer.
Amino Acid+ATP→Aminoacyl AMP+PPi
Transfer to tRNA:
The activated amino acid is transferred to the 3' hydroxyl group of the terminal adenosine (A76) of the tRNA. This forms a covalent ester bond between the amino acid's carboxyl group and the ribose sugar of A76.
Aminoacyl AMP+tRNA→Aminoacyl tRNA+AMP Proofreading (for certain aaRSs):
Some aaRSs have an editing domain that hydrolyzes incorrectly charged amino acids, ensuring fidelity in tRNA aminoacylation.
Engineering Biological Charging for Synthetic tRNAs
Biological charging is modified in synthetic biology to incorporate non standard amino acids (nsAAs). Key approaches include:
Engineering aaRS Active Sites: The binding pocket of aaRS is mutated to accommodate nsAAs with unique side chains. Example: Incorporating keto, azido, or photocrosslinkable groups.
Orthogonal Systems
An orthogonal tRNA aaRS pair is used, functioning independently of the host's natural translation system to prevent cross reactivity.
Example: Methanocaldococcus jannaschii tyrosyl tRNA synthetase (MjTyrRS) has been engineered to charge synthetic tRNAs with a variety of nsAAs.
Expanded Codon Recognition:
Synthetic tRNAs are designed to recognize reassigned codons (e.g., UAG or quadruplet codons), enabling site specific incorporation of nsAAs.
Advantages of Biological Charging
High Efficiency: Enzymes catalyze aminoacylation with high speed and specificity.
In Vivo Compatibility: Works seamlessly within living cells for protein synthesis.
Dynamic Regulation: The activity of aaRS can be controlled genetically or chemically.
Challenges of Biological Charging
Limited by the substrate scope of natural or engineered aaRSs.
Inefficient incorporation of bulky or chemically reactive nsAAs.
Competition with endogenous tRNA aaRS pairs.
Chemical Charging of tRNAs
Chemical charging bypasses the need for aaRSs by using non enzymatic methods to attach amino acids or nsAAs to tRNAs. This approach is particularly useful for incorporating amino acids that cannot be handled by biological systems.
Steps in Chemical Charging
tRNA Preparation: The tRNA is often truncated or chemically modified at its 3' end to expose a reactive group (e.g., hydroxyl or phosphate).
Amino Acid Activation:
The amino acid is chemically activated, typically as an N protected aminoacyl ester or iminium salt.
Common activating agents include: Carbodiimides (e.g., EDC, DCC). N Hydroxysuccinimide (NHS) esters.
Conjugation
The activated amino acid reacts with the 3' end of the tRNA, forming a covalent ester bond.
tRNA OH+Aminoacyl Ester→Aminoacyl tRNA
Purification:
Chemically charged tRNAs are purified to remove unreacted amino acids and byproducts.
Advantages of Chemical Charging
Substrate Flexibility: Chemically charged tRNAs can incorporate amino acids with virtually any chemical structure, including those with large, hydrophobic, or reactive groups.
Site Specific Labeling
Enables precise positioning of functional groups for protein modifications, such as fluorescent dyes or crosslinking agents.
In Vitro Applications
Ideal for cell free translation systems where chemically charged tRNAs can directly substitute for natural tRNAs.
Challenges of Chemical Charging
Technical Complexity: The synthesis of aminoacyl tRNA conjugates is labor intensive and requires precise chemical conditions.
Stability:
Ester bonds between the amino acid and tRNA are prone to hydrolysis, requiring careful handling and storage.
Inefficient Yield:
Chemical charging often produces lower yields compared to enzymatic charging, particularly for large scale applications.
Hybrid Systems
In some cases, chemical and biological charging are combined to achieve greater flexibility and efficiency:
In Vitro Priming: A tRNA is chemically pre charged with a reactive intermediate, which is then enzymatically converted to a final nsAA tRNA product. Example: Pre charging with a precursor amino acid that is post translationally modified by enzymes.
Chemical Augmentation of Biological Systems
Chemical modification of tRNAs or amino acids is used to improve the substrate scope of biological charging systems.
Applications of Charged tRNAs
Protein Engineering
Site specific incorporation of nsAAs with novel chemical, optical, or mechanical properties into proteins.
Example: Incorporating photocaged nsAAs for light controlled protein activity.
Therapeutics
Producing modified proteins or antibodies with enhanced stability or drug conjugation sites.
Example: Creating antibody drug conjugates with precisely positioned payloads.
Biophysical Studies
Site specific labeling of proteins for structural or interaction studies.
Example: Attaching fluorescent or spin label nsAAs for spectroscopy or microscopy.
Biological and chemical charging of tRNAs provide complementary strategies for incorporating standard and non standard amino acids into proteins. While biological charging excels in efficiency and in vivo applicability, chemical charging offers unmatched flexibility for handling diverse and complex amino acids. Together, these methods empower cutting edge applications in synthetic biology, protein engineering, and therapeutic development.
Applications of Synthetic tRNAs in Protein Engineering
Incorporation of Non Standard Amino Acids (nsAAs)
Synthetic tRNAs enable site specific incorporation of nsAAs with unique chemical properties, including:
Fluorescent nsAAs for protein tracking.
Photoreactive nsAAs for cross linking experiments.
Post translational mimicry through amino acids with phosphoryl, glycosyl, or acetyl groups.
2. Engineering Precise Protein Structures
Synthetic tRNAs allow precise amino acid positioning, creating proteins with
Defined bond geometries: nsAAs with specific steric or electronic properties control local folding and stability.
Cross linkable sites: Proteins can be covalently bonded at defined positions using nsAAs with reactive side chains.
Allosteric control: Incorporation of light sensitive nsAAs enables proteins to change conformation in response to specific wavelengths.
Example: Inserting p benzoyl L phenylalanine (a photoreactive nsAA) into an enzyme’s active site allows light controlled enzymatic activity.
De Novo Protein Design
By integrating synthetic tRNAs into artificial genetic circuits:
Entirely new protein architectures can be designed with no natural counterparts.
Proteins with mechanical properties, such as elasticity or self assembly, can be constructed by incorporating nsAAs into structural motifs.
Enhancing Therapeutic Proteins
Synthetic tRNAs expand the repertoire of modifications for biopharmaceuticals:
Improved drug delivery: Site specific conjugation of drugs to therapeutic antibodies.
Reduced immunogenicity: Incorporation of nsAAs that reduce recognition by immune cells.
Enhanced stability: Designing proteins resistant to proteolysis through strategically placed nsAAs.
Tools and Techniques for Synthetic tRNA Integration
Genetic Code Expansion Systems
Plasmid based expression: Synthetic tRNA and aaRS genes are delivered on plasmids.
Genome integration: Stable incorporation of synthetic tRNA machinery into the host genome for continuous use.
Ribosome Engineering
Modified ribosomes are often paired with synthetic tRNAs to efficiently translate expanded genetic codes:
Orthogonal Ribosomes (oRibs): Engineered ribosomes that specifically interact with synthetic tRNAs and not with endogenous tRNAs.
In Vitro Translation Systems
Cell free systems provide precise control over synthetic tRNA mediated protein synthesis:
Avoids competition with natural translation machinery.
Enables incorporation of nsAAs not tolerated in living organisms.
Challenges and Advances
Challenges
Efficiency: Low efficiency in charging synthetic tRNAs or in translating expanded genetic codes.
Host Toxicity: Overexpression of synthetic machinery can strain the host organism.
Codon Competition: Endogenous tRNAs may still compete for reassigned codons.
Advances
High Fidelity Systems: Improved orthogonal tRNA aaRS pairs with minimal off target effects.
Genetic Circuits: Synthetic circuits that dynamically regulate the expression of synthetic tRNAs.
Expanded Codon Space: Utilization of quadruplet codons or artificial nucleotides to further expand the genetic code.
Future Directions
Higher Order Protein Assemblies: Synthetic tRNAs can enable site specific incorporation of nsAAs to create protein hydrogels, nanostructures, or self assembling biomaterials.
Programmable Protein Behavior
Proteins with light , pH , or redox sensitive nsAAs for responsive therapeutic or catalytic functions.
Integration with AI
Machine learning can predict optimal positions for nsAAs to achieve desired structural or functional outcomes.
Conclusion
Synthetic tRNAs are a cornerstone of modern synthetic biology, enabling precise amino acid positioning to design unique protein structures with novel functionalities. Through innovations in genetic code expansion, orthogonal systems, and translation efficiency, synthetic tRNAs hold the promise of creating tailored proteins for applications ranging from therapeutics to material science. Continued development in this field will unlock further potential for custom designed biomolecules in diverse scientific and industrial contexts.
The integration of synthetic tRNAs into protein engineering represents a transformative leap in the field of synthetic biology. By enabling the incorporation of non standard amino acids (nsAAs) into proteins with site specific precision, synthetic tRNAs expand the natural genetic code, allowing researchers to design proteins with unprecedented structural and functional diversity. This capability has revolutionized the design of therapeutic proteins, enzymes, and biomaterials, facilitating applications that range from drug delivery and biopharmaceuticals to advanced bioelectronics and nanotechnology.
The core principles of synthetic tRNA engineering, including codon reassignment, orthogonal tRNA synthetase pairs, and expanded codon spaces, have provided a robust framework for overcoming the limitations of the canonical genetic code. Advances in both biological and chemical methods for charging tRNAs have further enhanced the scope and precision of nsAA incorporation, enabling the creation of proteins with unique chemical, mechanical, and optical properties. As discussed in this article, the development of orthogonal translation systems and the use of engineered ribosomes have significantly improved the fidelity and efficiency of these processes, paving the way for increasingly complex protein designs.
Despite these advancements, challenges such as codon competition, host system inefficiencies, and limited translation fidelity remain obstacles to the widespread application of synthetic tRNAs. However, ongoing innovations—such as orthogonal ribosomes, AI guided tRNA synthetase design, and cell free translation systems—are rapidly addressing these limitations. These emerging technologies, coupled with a deeper understanding of genetic code expansion, are expected to unlock the full potential of synthetic tRNAs, enabling the production of proteins that were once beyond the reach of natural systems.
Synthetic tRNAs are poised to become a cornerstone of modern biotechnology, driving innovations in synthetic biology and reshaping our ability to engineer life at the molecular level. As the field continues to evolve, the ability to create tailor made proteins with precise amino acid positioning will open new frontiers in science and industry, offering solutions to some of the most complex challenges in medicine, materials science, and beyond. Through the continued refinement of synthetic tRNA systems, the boundaries of what can be achieved in protein engineering will be redefined, ushering in a new era of molecular innovation.